Hydrogeology. Digital Solute-Transport Simulation of Nitrate and Geochemistry of Fluoride in Ground Waters of Cohanche County
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mountains, Streams, and Lakes of Oklahoma I
Information Series #1, June 1998 Mountains, Streams, and Lakes of OklahomaI Kenneth S. Johnson2 INTRODUCTION valleys, hills, and plains throughout most of the re mainder of Oklahoma (Fig. 1). All the major lakes and Mountains and streams define the landscape of reservoirs of Oklahoma are man-made, and they are Oklahoma (Fig. 1). The mountains consist mainly of important for flood contr()l, water supply, recreation, resistant rock masses that were folded, faulted, and and generation of hydroelectric power. Natural lakes thrust upward in the geologic past (Fig. 2), whereas in Oklahoma are limited to oxbow lakes along major the streams have persisted in eroding less-resistant streams and to playa lakes in the High Plains region rock units and lowering the landscape to form broad of the west. Alphabetical List of20 Lakes with Largest Surface Area (from Oklahoma Water Atlas, Oklahoma Water Resources Board) 1. Broken Bow 11. Lake 0' The Cherokees 2. Canton 12. Oologah 3. Eufaula 13. Robert s. Kerr 4. Fort Gibson 14. Sardis 5. Foss 15. Skiatook 6. Great Salt Plains 16. Tenkiller Ferry ·7. Hudson 17. Texoma 8. Hugo 18. Waurika 9. Kaw 19. Webbers Falls 10. Keystone 20. Wister Modified from Historical Atlas of Oklahoma, by John W. Morris, Charles R. Goins, and Edwin C. 25 McReynolds. Copyright © 1986 by the University I of Oklahoma Press. o 40 80Km Figure 1. Mountains, streams, and principal lakes of Oklahoma. lReprinted from Oklahoma Geology Notes (1993), vol. 53, no. 5, p. 180-188. The Notes article was reprinted and expanded from Oklahoma Almanac, 1993-1994, Oklahoma Department of Lihraries, p. -
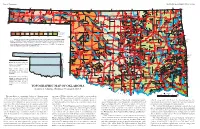
TOPOGRAPHIC MAP of OKLAHOMA Kenneth S
Page 2, Topographic EDUCATIONAL PUBLICATION 9: 2008 Contour lines (in feet) are generalized from U.S. Geological Survey topographic maps (scale, 1:250,000). Principal meridians and base lines (dotted black lines) are references for subdividing land into sections, townships, and ranges. Spot elevations ( feet) are given for select geographic features from detailed topographic maps (scale, 1:24,000). The geographic center of Oklahoma is just north of Oklahoma City. Dimensions of Oklahoma Distances: shown in miles (and kilometers), calculated by Myers and Vosburg (1964). Area: 69,919 square miles (181,090 square kilometers), or 44,748,000 acres (18,109,000 hectares). Geographic Center of Okla- homa: the point, just north of Oklahoma City, where you could “balance” the State, if it were completely flat (see topographic map). TOPOGRAPHIC MAP OF OKLAHOMA Kenneth S. Johnson, Oklahoma Geological Survey This map shows the topographic features of Oklahoma using tain ranges (Wichita, Arbuckle, and Ouachita) occur in southern contour lines, or lines of equal elevation above sea level. The high- Oklahoma, although mountainous and hilly areas exist in other parts est elevation (4,973 ft) in Oklahoma is on Black Mesa, in the north- of the State. The map on page 8 shows the geomorphic provinces The Ouachita (pronounced “Wa-she-tah”) Mountains in south- 2,568 ft, rising about 2,000 ft above the surrounding plains. The west corner of the Panhandle; the lowest elevation (287 ft) is where of Oklahoma and describes many of the geographic features men- eastern Oklahoma and western Arkansas is a curved belt of forested largest mountainous area in the region is the Sans Bois Mountains, Little River flows into Arkansas, near the southeast corner of the tioned below. -

Four Western Cheilanthoid Ferns in Oklahoma
Oklahoma Native Plant Record 65 Volume 10, December 2010 FOUR WESTERN CHEILANTHOID FERNS IN OKLAHOMA Bruce A. Smith McLoud High School McLoud, Oklahoma 74851 Keywords: arid, distribution, habitat, key ABSTRACT The diversity of ferns in some of the more arid climates of western Oklahoma is surprising. This article examines four Oklahoma cheilanthoid ferns: Astrolepis integerrima, Cheilanthes wootonii, Notholaena standleyi, and Pellaea wrightiana. With the exceptions of A. integerrima and P. wrightiana which occur in Alabama and North Carolina respectively, all four species reach their eastern limits of distribution in Oklahoma. Included in this article are common names, synonyms, brief descriptions, distinguishing characteristics, U.S. and Oklahoma distribution, habitat information, state abundance, and a dichotomous key to selected cheilanthoids. The Oklahoma Natural Heritage Inventory has determined that all but one (N. standleyi) are species of concern in the state. INTRODUCTION of eastern Oklahoma, while most members of the Pteridaceae occur in Almost half of the ferns in the family western Oklahoma (Taylor & Taylor Pteridaceae are xeric adapted ferns. In 1991). Oklahoma six genera and sixteen species Statewide, the most common species in the family are known to occur. They in the Pteridaceae is Pellaea atropurpurea live on dry or moist rocks and can be (Figure 4), which can be found found in rock crevices, at the bases of throughout the body of the state and boulders, or on rocky ledges. Common Cimarron County in the panhandle. The associated species include lichens, mosses, rarest are Cheilanthes horridula and liverworts, and spike mosses. Two Cheilanthes lindheimeri. Cheilanthes horridula physical characteristics that unite the and Cheilanthes lindheimeri have only been family are the marginal sori (Figure 1) and seen in one county each, Murray and the lack of a true indusium. -

Description of the Coalgate Quadrangle
' *' DESCRIPTION OF THE COALGATE QUADRANGLE. By Joseph A. Taff. GEOGRAPHY. forms of the Ouachita Mountain Range. The a long time the valleys become wide and silted, that at ordinary conditions the stream meanders ridges of the valley region are nearly parallel and the hills are gradually reduced to the level in rivulets or narrow channels. Indeed, its chan GENERAL RELATIONS. with those of the range, but, with the exception of the valleys. nel is so choked with sand that the water does The Coalgate quadrangle is bounded by the of the few isolated mountains which lie in Arkan The surface of the Coalgate quadrangle is of not at any stage of the river flow on the country meridians 96° and 96° 30' and the parallels 34° sas Valley, they have low relief. low relief, and the topographic features indicate rock. During floods, which usually come in 30' and 35°, and thus occupies one- Extentand The Prairie Plains region stretches from the that it has been so for a relatively long period of spring from the headwaters of the river, vast quarter of a square degree of the earth's th1?Juad=f Arkansas Valley and Ozark highland regions time. The larger streams have nearly ceased cut quantities of sands are swept down, shifting for surface. It is 34.4 miles long north rangle> northward and westward across north- Character ting their valleys deeper, and throughout most of mer shoals and channels. Little River, which is and south and 28.5 miles wide, and contains west Indian Territory into Oklahoma their courses are meandering in the deposits of silt tributary to the Canadian, crosses the northwest nearly 980 square miles. -

Ouachita Mountains Ecoregional Assessment December 2003
Ouachita Mountains Ecoregional Assessment December 2003 Ouachita Ecoregional Assessment Team Arkansas Field Office 601 North University Ave. Little Rock, AR 72205 Oklahoma Field Office 2727 East 21st Street Tulsa, OK 74114 Ouachita Mountains Ecoregional Assessment ii 12/2003 Table of Contents Ouachita Mountains Ecoregional Assessment............................................................................................................................i Table of Contents ........................................................................................................................................................................iii EXECUTIVE SUMMARY..............................................................................................................1 INTRODUCTION..........................................................................................................................3 BACKGROUND ...........................................................................................................................4 Ecoregional Boundary Delineation.............................................................................................................................................4 Geology..........................................................................................................................................................................................5 Soils................................................................................................................................................................................................6 -

Age of the Folding of the Oklahoma Mountains— % the Ouachita, Arbuckle, and Wichita Moun Tains of Oklahoma and the Llano-Burnet and Marathon Uplifts of Texas 1
BULLETIN OF THE GEOLOGICAL SOCIETY OF AMERICA Vol. 39. pp. 1031-1072 December 30. 1928 AGE OF THE FOLDING OF THE OKLAHOMA MOUNTAINS— % THE OUACHITA, ARBUCKLE, AND WICHITA MOUN TAINS OF OKLAHOMA AND THE LLANO-BURNET AND MARATHON UPLIFTS OF TEXAS 1 BY SIDNEY POWERS 2 (Presented by title before the Society December SI, 1927) CONTENTS Tage Introduction ............................................................................................................. 1031 Stratigraphy ............................................................................................................. 1036 The, Ouachita Mountains ..................................................................................... 1037 Problems............................................................................................................. 1037 Age of the Carboniferous sediments .......................................................... 1038 Origin of the “glacial” boulders .................................................................. 1042 Date of the folding and overthrusting ...................................................... 1047 The Arbuckle Mountains ........................................................................................ 1049 The Wichita Mountains ........................................................................................ 1056 Former extent ................................................................................................. 1056 Criner Hills .................................................................................................... -

Teacher's Guide
Destinations OklahomaTeacher's Guide Content for this educational program provided by: CIMC Students of All Ages: Your adventure is about to begin! Within these pages you will become a “Geo-Detective” exploring the six countries of Oklahoma. Yes, countries! Within Oklahoma you’ll be traveling to unique places or regions called “countries.” Maybe you’ve heard of “Green Country” with its forests and specialty crops, or “Red Carpet Country,” named for the red rocks and soil formed during the ancient Permian age. Each region or country you visit will have special interesting themes or features, plus fun and sometimes challenging activities that you will be able to do. You will notice each country or region can be identifi ed by natural, economic, historic, cultural, geographic and geological features. The three maps you see on this page are examples of maps you might need for future Geo-Explorations. As a Geo-Detective having fun with the following activities, you’ll experience being a geographer and a geologist at the same time! So for starters, visit these websites and enjoy your Geo-Adventure: http://education.usgs.gov http://www.ogs.ou.edu http://www.census.gov http://www.travelok.com/site/links.asp Gary Gress, Geographer Neil Suneson, Geologist Oklahoma Alliance for Geographic Education Oklahoma Geological Survey Teachers: PASS Standards met by Destinations Oklahoma are listed on pages 15 – 17. Indian Nations of Oklahoma 1889 - Before and after the Civil War, tribal boundaries were constantly changing due to U.S. government policies. Eventually the Eastern and Western tribes merged into a state called “Oklahoma,” meaning “(land of) red people.” Oklahoma's 10 Geographic Regions - These regions refl ect both physical features (topography) and soils. -

Arbuckle Project
Arbuckle Project Christopher J. McCune Historic Reclamation Projects Bureau of Reclamation 2002 Table of Contents Table of Contents..............................................................1 The Arbuckle Project...........................................................2 Project Location.........................................................2 Historic Setting .........................................................3 Project Authorization.....................................................5 Construction History .....................................................8 Post-Construction History................................................15 Settlement of the Project .................................................19 Uses of Project Water ...................................................20 Conclusion............................................................21 About the Author .............................................................21 Bibliography ................................................................22 Archival Collections ....................................................22 Government Documents .................................................22 Articles...............................................................22 Internet...............................................................22 Other ................................................................22 Index ......................................................................23 1 The Arbuckle Project In 1962, President John F. Kennedy, authorized -

Mountain Ranges INSIDE on PAGE 4 OKLAHOMA GEOLOGICAL SURVEY
VOLUME 76, NO. 4 OCTOBER-DECEMBER 2017 The Age of Oklahoma’s Mountain Ranges INSIDE ON PAGE 4 OKLAHOMA GEOLOGICAL SURVEY OKLAHOMA GEOLOGICAL SURVEY DR. JEREMY BOAK, Director Editor OGS geologists Neil Suneson and Ted Satterfield Thomas Stanley provide answers to the question: “How old are Oklahoma’s mountain ranges?” — Page 4 Cartography Manager James Anderson GIS Specialist Russell Standridge Copy Center Manager Richard Murray This publication, printed by the Oklahoma Geological Survey, Norman, Oklahoma, is issued by the Oklahoma Geological Survey as authorized by Title 70, Oklahoma Statutes 1981, Section 3310, and Title 74, Oklahoma Statutes 1981, Sections 231—238 The Oklahoma Geological Survey is a state agency for research and public service, mandated in the State Constitution to study Oklahoma’s land, water, mineral and energy resources and to promote wise Cover: Photo was shot on the Talimena Drive, which stretches across the Ouachita Mountains use and sound environmental practices. in southeastern Oklahoma Photo and Cover Design by Ted Satterfield 2 OCTOBER-DECEMBER 2017 Mountain ranges, a new hire, and a new year This issue focuses on a seemingly very straightforward question, one that all kinds of thought ful people, experts and lay people, like to ask, and ex pect a straightforward answer: how old are Oklahoma’s mountains? The review by Neil Suneson and Tom Stanley highlights the extent to which, even for this simple question, the answer can be quite complicated, and, in some places, the answer may still be “We don’t know.” Even for people like Neil and Tom, who have been working on Oklahoma geology for a long time and have a breadth of knowledge that we constantly call on, there are still aspects of a problem like this that can fall in the gap where they have not worked or have not yet come to a solid answer. -

Geologic Map of Chickasaw National Recreation Area, Murray County, Oklahoma
Prepared in cooperation with the National Park Service Geologic Map of Chickasaw National Recreation Area, Murray County, Oklahoma Pamphlet to accompany Scientific Investigations Map 3258 U.S. Department of the Interior U.S. Geological Survey 1 2 3 4 Cover photographs: 1 Massive sandstone, Vanoss Formation (*vs), see fig. 5. 2 Old abandoned quarry exposing Hunton Group (DSOh) and crest of Tishomingo anticline in southeastern part of map area (looking east-southest), see map sheet. 3 Lower Hunton Group (SOhl, Cochrane Formation), see fig. 4. 4 Woodford Shale (MDsw), see fig. 5. Geologic Map of Chickasaw National Recreation Area, Murray County, Oklahoma By Charles D. Blome, David J. Lidke, Ronald R. Wahl, and James A. Golab Prepared in cooperation with the National Park Service Pamphlet to accompany Scientific Investigations Map 3258 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior SALLY JEWELL, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2013 Revised May 8, 2014 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. -

Anorthosite-Gabbro-Granophyte Relationships, Mount Sheridan Area
RICE UNIVERSITY ANORTHOSITE-GABBRO-GRANOPHYRE RELATIONSHIPS, MOUNT SHERIDAN AREA, OKLAHOMA by EDWARD C. THORNTON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS Thesis Director's Signature: Houston, Texas May, 1975 ABSTRACT ANORTHOSITE-GABBRO-GRANOPHYRE RELATIONSHIPS MOUNT SHERIDAN AREA, OKLAHOMA Edward C. Thornton Field work, phase analysis by microprobe, petrography, and oxygen isotope analysis have been undertaken in an ef¬ fort to establish the relationships among the anorthositic, gabbroic, and granophyric rocks of the Mount Sheridan area in southwestern Oklahoma. The gabbro has been found to be somewhat finer-grained when in contact with the anorthosite. Petrographically, the gabbro is quite different from the anorthosite. The anorthosite is essentially a one-pyroxene rock (clinopy- roxene) while the gabbro contains both augite and hyper- sthene. Quartz, micropegmatite, and biotite are present in the gabbro but essentially absent in the anorthosite. The anorthosite is a cumulate rock with well-developed igneous lamination whereas the gabbro is generally not laminated. The above information established that the anorthosite is the older unit and that the gabbro was sub¬ sequently intruded into it. The gabbro is transitional into the overlying grano- phyre through a zone of intermediate rock. The zone of transition has been found to extend into the gabbro itself as has been determined by phase analysis, in addition, diabasic inclusions are present in the granophyre - evi¬ dence of possible mixing of basaltic and granophyric magma. These observations suggest that gabbroic and granophyric magmas were emplaced simultaneously at Mount Sheridan and that the intermediate rock between the gabbro and grano¬ phyre may have been generated by mixing of the two magma types. -

The Mount Scott Intrusive Suite, Wichita Mountains, Oklahoma
The Mount Scott Intrusive Suite, Wichita Mountains, Oklahoma Jonathan D. Price Department of Chemistry, Geosciences, and Physics, Midwestern State University, 3410 Taft Boulevard, Wichita Falls, Texas, 76308. [email protected]. ph. (940) 397-4288. ABSTRACT blende and alkali feldspar near its top to produce a liq- uid that gave rise to the Saddle Mountain Granite. In the last 50 years the Mount Scott Granite and three other granite bodies—the Rush Lake Granite, INTRODUCTION the Medicine Park Granite, and the Saddle Moun- tain Granite—have been characterized and mapped Nearly half a century has passed since Clifford A. Mer- as separate lithodemic units within the eastern Wich- ritt recognized the Mount Scott Granite as a distinctive unit ita Mountains of southwestern Oklahoma. These are based on its petrographic characteristics (Merritt, 1965). part of the Wichita Granite Group (WGG), products His paper is a classic examination of this well-exposed of felsic magmatism during Eocambrian rifting of the Cambrian granite in the eastern Wichita Mountains, south- Southern Oklahoma Aulacogen. Like all members of western Oklahoma. His careful examination arose from the WGG, these are pink-to-red A-type alkali feldspar the exhaustive seminal study of Oklahoma’s crystalline granites. Each is porphyritic and wholly or partially rocks undertaken with William E. Ham and Roger E. Den- granophyric, as are other WGG members. These units, ison (Ham et al., 1964) published the previous year. Ham however, share contact and compositional relationships et al. (1964) examined the rocks of the Wichita Mountains that suggest a common reservoir in the crust that ulti- as the surface exposure of earliest Cambrian magmatism mately fed shallow (locally subvolcanic) tabular pluton- preserved in the Oklahoma basement.