USAID ERADICATE TB PROJECT QUARTERLY PROGRESS REPORT Reporting Period: January 1 to March 31, 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zambia Country Operational Plan (COP) 2016 Strategic Direction Summary
Zambia Country Operational Plan (COP) 2016 Strategic Direction Summary June 14, 2016 Table of Contents Goal Statement 1.0 Epidemic, Response, and Program Context 1.1 Summary statistics, disease burden and epidemic profile 1.2 Investment profile 1.3 Sustainability profile 1.4 Alignment of PEPFAR investments geographically to burden of disease 1.5 Stakeholder engagement 2.0 Core, near-core and non-core activities for operating cycle 3.0 Geographic and population prioritization 4.0 Program Activities for Epidemic Control in Scale-up Locations and Populations 4.1 Targets for scale-up locations and populations 4.2 Priority population prevention 4.3 Voluntary medical male circumcision (VMMC) 4.4 Preventing mother-to-child transmission (PMTCT) 4.5 HIV testing and counseling (HTS) 4.6 Facility and community-based care and support 4.7 TB/HIV 4.8 Adult treatment 4.9 Pediatric treatment 4.10 Orphans and vulnerable children (OVC) 5.0 Program Activities in Sustained Support Locations and Populations 5.1 Package of services and expected volume in sustained support locations and populations 5.2 Transition plans for redirecting PEPFAR support to scale-up locations and populations 6.0 Program Support Necessary to Achieve Sustained Epidemic Control 6.1 Critical systems investments for achieving key programmatic gaps 6.2 Critical systems investments for achieving priority policies 6.3 Proposed system investments outside of programmatic gaps and priority policies 7.0 USG Management, Operations and Staffing Plan to Achieve Stated Goals Appendix A- Core, Near-core, Non-core Matrix Appendix B- Budget Profile and Resource Projections 2 Goal Statement Along with the Government of the Republic of Zambia (GRZ), the U.S. -

Fostering Accountability and Transparency (FACT) in Zambia Quarterly Report
Fostering Accountability and Transparency (FACT) in Zambia Quarterly Report January 1 to March 30, 2019 Youth Symposium Participants Outside FQM Trident Foundation Limited Offices after receiving training from one of FACT partners Submission Date: April 30, 2019 Submitted by: Chilufya Kasutu Agreement Number: Chief of Party AID-611-14-L-00001 Counterpart International, Zambia Email: [email protected] Submitted to: Edward DeMarco, USAID Zambia AOR This document was produced for review by the United States Agency for International Development, Zambia (USAID/Zambia). It was prepared by Counterpart International. ACRONYMS AND ABBREVIATIONS AOR Agreement Officer’s Representative ART Anti-Retroviral Treatment CCAs Community Conservation Areas CCPs Community Conservation Plans CFGs Community Forest Groups CEFTA Citizens Engagement in Fostering Transparency and Accountability COMACO Community Markets for Conservation CRB Community Resource Boards CSPR Civil Society for Poverty Reduction CSO Civil Society Organization DAC District Advocacy Committee DAMI District Alternative Mining Indaba DDCC District Development Coordinating Committee DEBS District Education Board Secretary DHO District Health Office DIM District Integrated Meetings EITI Extractive Industries Transparency Initiative ESSP Education and Skills Sector Plan FACT Fostering Accountability and Transparency FZS Frankfurt Zoological Society GPE Global Partnership for Education GRZ Government of the Republic of Zambia HCC Health Centre Committee HIV Human Immunodeficiency Virus LAG -

Environmental Project Brief
Public Disclosure Authorized IMPROVED RURAL CONNECTIVITY Public Disclosure Authorized PROJECT (IRCP) REHABILITATION OF PRIMARY FEEDER ROADS IN EASTERN PROVINCE Public Disclosure Authorized ENVIRONMENTAL PROJECT BRIEF September 2020 SUBMITTED BY EASTCONSULT/DASAN CONSULT - JV Public Disclosure Authorized Improved Rural Connectivity Project Environmental Project Brief for the Rehabilitation of Primary Feeder Roads in Eastern Province Improved Rural Connectivity Project (IRCP) Rehabilitation of Primary Feeder Roads in Eastern Province EXECUTIVE SUMMARY The Government of the Republic Zambia (GRZ) is seeking to increase efficiency and effectiveness of the management and maintenance of the of the Primary Feeder Roads (PFR) network. This is further motivated by the recognition that the road network constitutes the single largest asset owned by the Government, and a less than optimal system of the management and maintenance of that asset generally results in huge losses for the national economy. In order to ensure management and maintenance of the PFR, the government is introducing the OPRC concept. The OPRC is a concept is a contracting approach in which the service provider is paid not for ‘inputs’ but rather for the results of the work executed under the contract i.e. the service provider’s performance under the contract. The initial phase of the project, supported by the World Bank will be implementing the Improved Rural Connectivity Project (IRCP) in some selected districts of Central, Eastern, Northern, Luapula, Southern and Muchinga Provinces. The project will be implemented in Eastern Province for a period of five (5) years from 2020 to 2025 using the Output and Performance Road Contract (OPRC) approach. GRZ thus intends to roll out the OPRC on the PFR Network covering a total of 14,333Kms country-wide. -

Profiles of Active Civil Society Organisations in North-Western, Copperbelt and Southern Provinces of Zambia
Profiles of Active Civil Society Organisations in North-Western, Copperbelt and Southern Provinces of Zambia On behalf of Implemented by Published by: Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH Registered offices Bonn and Eschborn, Germany Address Civil Society Participation Programme (CSPP) Mpile Office Park, 3rd floor 74 Independence Avenue Lusaka, Zambia P +260 211 250 894 E [email protected] I www.giz.de/en Programme: Civil society participation in governance reform and poverty reduction Author: Isaac Ngoma, GFA Consulting Group GmbH Editor: Markus Zwenke, GFA Consulting Group GmbH, Eulenkrugstraße 82, 22359 Hamburg, Germany Design/layout: GFA Consulting Group GmbH and IE Zhdanovich Photo credits/sources: GFA Consulting Group GmbH On behalf of German Federal Ministry for Economic Cooperation and Development (BMZ) As of June, 2021 TABLE OF CONTENT ACTIVE CIVIL SOCIETY ORGANISATIONS IN NORTH-WESTERN PROVINCE � � � � � �7 Dream Achievers Academy �������������������������������������������������������������������������������������������������������������������������� 8 Anti-voter Apathy Project ���������������������������������������������������������������������������������������������������������������������������� 9 Mentra Youth Zambia . 10 The Africa Youth Initiative Network �������������������������������������������������������������������������������������������������������� 11 Radio Kabangabanga ���������������������������������������������������������������������������������������������������������������������������������� -

Ministerial Statement on the 2020/2021 Rainy Season Forecast by the Hon. Minister of Transport and Communication
MINISTERIAL STATEMENT ON THE 2020/2021 RAINY SEASON FORECAST BY THE HON. MINISTER OF TRANSPORT AND COMMUNICATION (MR KAFWAYA), MP Mr Speaker, I thank you for giving me this opportunity to make a ministerial statement on the 2020/2021 Rainy Season Forecast. I feel duty bound to give this statement as whether and climate conditions affect all sectors of the economy across the country. Sir, over time, we have observed the climate change as a severe impact on many social-economic sectors of our country and the same could be said for the future. Therefore, the importance of weather information for decision making across relevant sectors is critical. More importantly, it is essential to make reasonably accurate whether and climate forecast in a timely manner. Accordingly, the Government under the able leadership of His Excellency Dr Edgar Chagwa Lungu, President of the Republic of Zambia, we have continued to equip the Zambia Metrological Department. This is being done through the procurement and installation of modern automatic data collection equipment to aid the process of whether forecasting. I would like to register my appreciation and that of the Government for the invaluable contribution from cooperating partners in providing equipment and funding for the success of this program. Mr Speaker, I wish to remind this august House that when the Patriotic (PF) Government came into power in 2011, there were only thirty-nine manual weather stations across the country. I wish to remind this august House that when the PF came into power in 2011, there were only thirty-nine manual whether stations across the country. -

THE RURAL ELECTRIFICATION AUTHORITY Invitation for Bids (IFB)
THE RURAL ELECTRIFICATION AUTHORITY Invitation for Bids (IFB) TENDER FOR THE SUPPLY, INSTALLATION AND COMMISSIONING OF 2018 GRID EXTENSION PROJECTS UNDER THE RURAL ELECTRIFICATION PROGRAMME IN SELECTED PROVINCES OF ZAMBIA - REA/ONB/W/2/2018 1. The Rural Electrification Authority has received financing from the Government of Zambia towards the cost of the implementation of Rural Electrification programme and intends to apply part of the financing to payments under the contracts for the supply, installation and commissioning of 2018 grid extension projects. 2. The Rural Electrification Authority now invites sealed bids from eligible and qualified bidders for the supply, installation and commissioning of 2018 grid extension projects in the following selected provinces of Zambia: LOT PROJECT NAME DISTRICT PROVINCE COMPLETION PERIOD 1 Mwenzo-Nawaitwika Nakonde Muchinga 40 weeks 2 Ukwimi Petauke Eastern 40 weeks 3 Kaulembe Chipata Eastern 40 weeks 4 Shemu Nakonde Muchinga 40 weeks 5 Musanya Chinsali Muchinga 40 weeks 6 Nangweshi Sioma Western 40 weeks 7 Sandwe Petauke Eastern 40 weeks 8 Jimbe Pemba Southern 40 weeks 9 Kaingu-Iyanda Segment 1 40 weeks Itezhi tezhi Central Kaingu-Iyanda Segment 2 10 Miponda-Shikamushile Samfya Luapula 40 weeks 11 Kalungu-Sasamwenge 40 weeks Segment 1 Isoka Muchinga Kalungu-Sasamwenge Segment 2 12 Lambwe Chomba Chiengi Luapula 40 weeks Lupososhi Segment 1 40 weeks Luwingu Northern 13 Lupososhi Segment 2 14 40 weeks Luano Phase I Segment 1 Luano Central Luano Phase I Segment 2 15 Luswishi Farm Block 40 weeks Segment 1 Lufwanyama Copperbelt Luswishi Farm Block Segment 2 16 Dundumwezi Phase I 40 weeks Segment 1 Kaloma Southern Dundumwezi Phase I Segment 2 17 Luembe Nyimba Eastern 40 weeks 18 Mutono-Chisheta Nchelenge/Mwense Luapula 40 weeks 19 Lwela-Milambo Segment 40 weeks 1 Milenge/Chembe Luapula Lwela-Milambo Segment 2 20 Mutashi Mufumbwe NorthWestern 40 weeks Bidders may submit a bid for one or more Lots. -
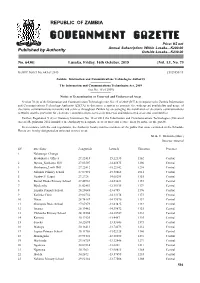
C:\Users\Public\Documents\GP JOBS\Gazette No. 73 of Friday, 16Th
REPUBLIC OF ZAMBIA Price: K5 net Annual Subscription: Within Lusaka—K200.00 Published by Authority Outside Lusaka—K230.00 No. 6430] Lusaka, Friday, 16th October, 2015 [Vol. LI, No. 73 GAZETTE NOTICE NO. 643 OF 2015 [5929855/13 Zambia Information and Communications Technologies Authority The Information and Communications Technologies Act, 2009 (Act No. 15 of 2009) Notice of Determination of Unserved and Underserved Areas Section 70 (2) of the Information and Communication TechnologiesAct No. 15 of 2009 (ICTAct) empowers the Zambia Information and Communications Technology Authority (ZICTA) to determine a system to promote the widespread availability and usage of electronic communications networks and services throughout Zambia by encouraging the installation of electronic communications networks and the provision for electronic communications services in unserved and underserved areas and communities. Further, Regulation 5 (2) of Statutory Instrument No. 38 of 2012 the Information and Communications Technologies (Universal Access) Regulations 2012 mandates the Authority to designate areas as universal service areas by notice in the gazette. In accordance with the said regulations, the Authority hereby notifies members of the public that areas contained in the Schedule Hereto are hereby designated as universal service areas. M. K. C. MUDENDA (MRS.) Director General SN Site Name Longtitude Latitude Elevation Province 1 Nalusanga_Chunga Headquarter Offices 27.22415 -15.22135 1162 Central 2 Mpusu_KankamoHill 27.03507 -14.45675 1206 Central -

CCMG Statement on Analysis of the Voters Register 14Apr21
CCMG Statement on Analysis of the Voters Register Understanding the Credibility and Acceptability of the Voters Register 14 April 2021 1. Preamble The Christian Churches Monitoring Group (CCMG) has completed its independent analysis of the voter registration numbers released by the Electoral Commission of Zambia (ECZ) in February 2021. CCMG commends the ECZ for releasing the voter registration figures showing the total number of voters registered, disaggregated by province, district and constituency, as well as gender. CCMG, using the voting age population (VAP) estimates from the Zambia Statistics Agency (ZamStats) that were released in December last year1, analysed the 2021 voter registration figures. The VAP is an estimate that shows how many eligible voters are estimated to reside in a given area. The use of VAP estimates to assess the quality of a voters register in terms of how well it reflects a country’s population is a standard practice of election observation groups worldwide. This is done to contribute to the credibility of the register because it determines how representative the register is of those who are eligible to vote. 2. Analysis The table below provides the results of the analysis of the announced registration figures by province compared to the ZamStats VAP estimates. It is important to note that while registration rates may not be identical across the country, they should be relatively uniform. Similar registration rates suggest that all eligible voters had an equal opportunity to register while significant disparities suggest under or over registration of particular communities. Province ECZ Registered Voters ZamStats VAP Estimate % Registered Luapula 562,230 601,058 93.5% Eastern 896,213 973,790 92.0% Western 447,143 507,184 88.2% North-Western 384,452 447,661 85.9% Northern 600,859 706,495 85.0% Total Zambia 7,002,393 8,414,839 83.2% Copperbelt 1,023,223 1,257,460 81.4% 1 CCMG sent a letter to ZamStats seeking clarification on how the VAP estimates were developed but has not yet received a response. -

Republic of Zambia Report of the Committee on Delegated Legislation For
REPUBLIC OF ZAMBIA REPORT OF THE COMMITTEE ON DELEGATED LEGISLATION FOR THE FIRST SESSION OF THE TWELFTH NATIONAL ASSEMBLY APPOINTED ON WEDNESDAY, 5TH OCTOBER, 2016 Printed by the National Assembly of Zambia REPORT OF THE COMMITTEE ON DELEGATED LEGISLATION FOR THE FIRST SESSION OF THE TWELFTH NATIONAL ASSEMBLY APPOINTED ON WEDNESDAY, 5TH OCTOBER, 2016 TABLE OF CONTENTS No. Paragraph Page 1. Composition of the Committee 1 2. Functions of the Committee 1 3. Meetings of the Committee 2 PART I – CONSIDERATION OF STATUTORY INSTRUMENTS Electoral Commission of Zambia 4. Statutory Instrument No. 33 of 2016 - The Electoral (Electoral Timetable ) 2 Order, 2016 5. Statutory Instrument No. 34 of 2016 – The Local Government Elections 3 (Elections Dates and Times of Poll) Order, 2016 6. Statutory Instrument No. 60 of 2016 – The Local Government Elections 3 Tribunals Rules of 2016 7. Statutory Instrument No. 62 of 2016 – The Electoral Process (Code of 3 Conduct) (Enforcement) Regulations, 2016 8. Statutory Instrument No. 63 of 2016 – The Electoral Process ( General) 3 Regulations, 2016 9. Statutory Instrument No. 64 of 2016 – The Referendum Regulations 2016 3 10. Statutory Instrument No. 18 of 2017 – The Local Government By-Election 4 (Election Dates and Times of Poll) Order, 2017 Ministry of Health 11. Statutory Instrument No. 10 of 2016 – The Medicines and Allied 4 Substances (Agro-Veterinary Shops) Regulations, 2016 12. Statutory Instrument No. 11 of 2016 - The Medicines and Allied 4 Substances (Dispensing Certificates) Regulation, 2016 13. Statutory Instrument No. 12 of 2016 - The Medicines and Allied 4 Substances (Health Shops) Regulations, 2016 14. Statutory Instrument No. -

Electoral Violence and Young Party Cadres in Zambia
VOLUME 18 NO 1 DOI: 10.20940/JAE/2019/v18i1aDOI: 10.20940/JAE/2019/v18i1a7 7 129 ELECTORAL VIOLENCE AND YOUNG PARTY CADRES IN ZAMBIA Kabale Ignatius Mukunto Kabale Ignatius Mukunto is a lecturer at the Dag Hammarskjöld Institute for Peace and Conflict Studies, Copperbelt University, Kitwe, Zambia ABSTRACT Zambia’s 2016 general elections were a turning point in the country’s political history, with electoral violence threatening its democratic fabric. This paper analyses accounts of electoral campaigns by one private online newspaper, the Lusaka Times, to reflect on the relationship between electoral violence and young party cadres. Evidence from the study indicates that negative socioeconomic conditions, leadership manipulation and incentives as well as the perception of plural politics all contribute to the susceptibility of young people to electoral violence. The violence witnessed in 2016 included molestation and intimidation, seizure of public property, public disorder, vandalising of party property, lawlessness and aggressive rhetoric. The paper also notes that events of 2016 were counterweight to the consolidation of democracy as the activities of young party cadres undermined the free political participation of other stakeholders. Keywords: electoral violence, electoral campaigns, young party cadres, Zambia INTRODUCTION Ascent to power through an election is assumed to legitimise those in government. Elections are considered a neutral arbiter for the contending parties and to some extent instil confidence in political players. Conversely, voters see considerable incentive in being part of the process to usher in their preferred candidates to facilitate improved service delivery. Elections constitute a transitional mechanism at the core of democracy, ideally supporting all institutions. -

Registered Voters by Gender and Constituency
REGISTERED VOTERS BY GENDER AND CONSTITUENCY % OF % OF SUB % OF PROVINCIAL CONSTITUENCY NAME MALES MALES FEMALES FEMALES TOTAL TOTAL KATUBA 25,040 46.6% 28,746 53.4% 53,786 8.1% KEEMBE 23,580 48.1% 25,453 51.9% 49,033 7.4% CHISAMBA 19,289 47.5% 21,343 52.5% 40,632 6.1% CHITAMBO 11,720 44.1% 14,879 55.9% 26,599 4.0% ITEZH-ITEZHI 18,713 47.2% 20,928 52.8% 39,641 5.9% BWACHA 24,749 48.1% 26,707 51.9% 51,456 7.7% KABWE CENTRAL 31,504 47.4% 34,993 52.6% 66,497 10.0% KAPIRI MPOSHI 41,947 46.7% 47,905 53.3% 89,852 13.5% MKUSHI SOUTH 10,797 47.3% 12,017 52.7% 22,814 3.4% MKUSHI NORTH 26,983 49.5% 27,504 50.5% 54,487 8.2% MUMBWA 23,494 47.9% 25,545 52.1% 49,039 7.4% NANGOMA 12,487 47.4% 13,864 52.6% 26,351 4.0% LUFUBU 5,491 48.1% 5,920 51.9% 11,411 1.7% MUCHINGA 10,072 49.7% 10,200 50.3% 20,272 3.0% SERENJE 14,415 48.5% 15,313 51.5% 29,728 4.5% MWEMBEZHI 16,756 47.9% 18,246 52.1% 35,002 5.3% 317,037 47.6% 349,563 52.4% 666,600 100.0% % OF % OF SUB % OF PROVINCIAL CONSTITUENCY NAME MALES MALES FEMALES FEMALES TOTAL TOTAL CHILILABOMBWE 28,058 51.1% 26,835 48.9% 54,893 5.4% CHINGOLA 34,695 49.7% 35,098 50.3% 69,793 6.8% NCHANGA 23,622 50.0% 23,654 50.0% 47,276 4.6% KALULUSHI 32,683 50.1% 32,614 49.9% 65,297 6.4% CHIMWEMWE 29,370 48.7% 30,953 51.3% 60,323 5.9% KAMFINSA 24,282 51.1% 23,214 48.9% 47,496 4.6% KWACHA 31,637 49.3% 32,508 50.7% 64,145 6.3% NKANA 27,595 51.9% 25,562 48.1% 53,157 5.2% WUSAKILE 23,206 50.5% 22,787 49.5% 45,993 4.5% LUANSHYA 26,658 49.5% 27,225 50.5% 53,883 5.3% ROAN 15,921 50.1% 15,880 49.9% 31,801 3.1% LUFWANYAMA 18,023 50.2% -

Members of the Northern Rhodesia Legislative Council and National Assembly of Zambia, 1924-2021
NATIONAL ASSEMBLY OF ZAMBIA Parliament Buildings P.O Box 31299 Lusaka www.parliament.gov.zm MEMBERS OF THE NORTHERN RHODESIA LEGISLATIVE COUNCIL AND NATIONAL ASSEMBLY OF ZAMBIA, 1924-2021 FIRST EDITION, 2021 TABLE OF CONTENTS FOREWORD ................................................................................................................................................ 3 PREFACE ..................................................................................................................................................... 4 ACKNOWLEDGEMENTS .......................................................................................................................... 5 ABBREVIATIONS ...................................................................................................................................... 7 INTRODUCTION ........................................................................................................................................ 9 PART A: MEMBERS OF THE LEGISLATIVE COUNCIL, 1924 - 1964 ............................................... 10 PRIME MINISTERS OF THE FEDERATION OF RHODESIA .......................................................... 12 GOVERNORS OF NORTHERN RHODESIA AND PRESIDING OFFICERS OF THE LEGISTRATIVE COUNCIL (LEGICO) ............................................................................................... 13 SPEAKERS OF THE LEGISTRATIVE COUNCIL (LEGICO) - 1948 TO 1964 ................................. 16 DEPUTY SPEAKERS OF THE LEGICO 1948 TO 1964 ....................................................................