Video-Assisted Thoracoscopic Surgery (VATS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Point of the Needle. Occult Pneumothorax: a Review P Gilligan, D Hegarty, T B Hassan
293 CASE REPORTS Emerg Med J: first published as 10.1136/emj.20.3.296 on 1 May 2003. Downloaded from The point of the needle. Occult pneumothorax: a review P Gilligan, D Hegarty, T B Hassan ............................................................................................................................. Emerg Med J 2003;20:293–296 maximal resonance, which was the left sixth intercostal space The case of a patient with an unusual medical condition in the anterior axillary line. Some 300 ml of air was aspirated and an occult pneumothorax is presented. The evidence from the left hemithorax and the patient clinically improved. for management of occult pneumothorax particularly in The chest radiograph revealed bilateral infiltrates and under- patients with underlying lung disease is reviewed and solu- lying cystic and bullous disease but failed to reveal evidence of tions to the acute clinical problems that may arise are a pneumothorax (fig 1). A chest radiograph performed after suggested. the needle decompression also failed to show a pneumotho- rax. Computed tomography (CT) of the thorax revealed an anterior pneumothorax (fig 2). This was drained under CT guidance by the placement of a chest drain catheter. 27 year old man with histiocytosis X presented to the During the patient’s in hospital stay his chest drain was emergency department with left posterior chest wall removed as his chest radiograph showed no evidence of Apain and marked dyspnoea. The patient previously had residual pneumothorax. The patient became markedly dysp- recurrent pneumothoraces, eight on the right and two on the noeic within 24 hours. Because of the clinical impression of left. He had undergone pleurodesis of the right lung. -

Patient; but by the Use of X-Rays, Bronchoscopy, and Exploratory Thoracotomy, We Are Beginning to Get a Conception of the Pathology in the Living, Which Is An
Postgrad Med J: first published as 10.1136/pgmj.11.111.25 on 1 January 1935. Downloaded from January, 1935 INTRATHORACIC NEOPLASMS 25 INTRATHORACIC NEOPLASMS By H. P. NELSON, M.A., M.D., F.R.C.S. (Assistant Surgeon, Brompton Hospital.) Primary neoplasms within the thorax fall into two main groups; those arising in the broncho-pulmonary system, which express themselves by the typical respira- tory symptoms of cough, sputum and haemoptysis; and mediastinal neoplasms, a heterogeneous group, which sooner or later draw attention to themselves by pressure on nerves or obstruction of veins, trachea or oesophagus. Broncho-Pulmonary Neoplasms. The growth usually starts in one of the larger bronchi. The outstanding symptoms are cough and hamoptysis and the physical signs are due to bronchial obstruction. Practically speaking, all this group are carcinomas, but recently bronchial adenomas have been recognized as a group which were previously often classified as malignant. Other innocent tumours such as fibromas, chondromas and papillomas have been reported. Bronchial Carcinomas are definitely on the increase; although some authorities still believe that this is only apparent owing to improved diagnostic methods, it by copyright. is difficult to maintain this view when the post-mortem records also indicate an increase. Our understanding of the gross pathology of these tumours is derived from the study of post-mortem material, when the condition has advanced to kill the patient; but by the use of X-rays, bronchoscopy, and exploratory thoracotomy, we are beginning to get a conception of the pathology in the living, which is an essential preliminary to treatment. -

Tracheal Intubation Following Traumatic Injury)
CLINICAL MANAGEMENT UPDATE The Journal of TRAUMA Injury, Infection, and Critical Care Guidelines for Emergency Tracheal Intubation Immediately after Traumatic Injury C. Michael Dunham, MD, Robert D. Barraco, MD, David E. Clark, MD, Brian J. Daley, MD, Frank E. Davis III, MD, Michael A. Gibbs, MD, Thomas Knuth, MD, Peter B. Letarte, MD, Fred A. Luchette, MD, Laurel Omert, MD, Leonard J. Weireter, MD, and Charles E. Wiles III, MD for the EAST Practice Management Guidelines Work Group J Trauma. 2003;55:162–179. REFERRALS TO THE EAST WEB SITE and impaired laryngeal reflexes are nonhypercarbic hypox- Because of the large size of the guidelines, specific emia and aspiration, respectively. Airway obstruction can sections have been deleted from this article, but are available occur with cervical spine injury, severe cognitive impairment on the Eastern Association for the Surgery of Trauma (EAST) (Glasgow Coma Scale [GCS] score Յ 8), severe neck injury, Web site (www.east.org/trauma practice guidelines/Emergency severe maxillofacial injury, or smoke inhalation. Hypoventi- Tracheal Intubation Following Traumatic Injury). lation can be found with airway obstruction, cardiac arrest, severe cognitive impairment, or cervical spinal cord injury. I. STATEMENT OF THE PROBLEM Aspiration is likely to occur with cardiac arrest, severe cog- ypoxia and obstruction of the airway are linked to nitive impairment, or severe maxillofacial injury. A major preventable and potentially preventable acute trauma clinical concern with thoracic injury is the development of Hdeaths.1–4 There is substantial documentation that hyp- nonhypercarbic hypoxemia. Lung injury and nonhypercarbic oxia is common in severe brain injury and worsens neuro- hypoxemia are also potential sequelae of aspiration. -

Thoracoscopy for Spontaneous Pneumothorax
Journal of Clinical Medicine Review Thoracoscopy for Spontaneous Pneumothorax José M. Porcel 1,2,3,* and Pyng Lee 4 1 Pleural Medicine Unit, Department of Internal Medicine, Hospital Universitari Arnau de Vilanova, 25198 Lleida, Spain 2 Institut de Recerca Biomèdica de Lleida Fundació Dr. Pifarré, IRBLleida, 25198 Lleida, Spain 3 School of Medicine, Universitat de Lleida, 25008 Lleida, Spain 4 Division of Respiratory and Critical Care Medicine, The National University Hospital, Singapore 119228, Singapore; [email protected] * Correspondence: [email protected] Abstract: Video-assisted thoracic surgery (VATS) is the treatment of choice for recurrence preven- tion in patients with spontaneous pneumothorax (SP). Although the optimal surgical technique is uncertain, bullous resection using staplers in combination with mechanical pleurodesis, chemical pleurodesis and/or staple line coverage is usually undertaken. Currently, patient satisfaction, post- operative pain and other perioperative parameters have significantly improved with advancements in thoracoscopic technology, which include uniportal, needlescopic and nonintubated VATS variants. Ipsilateral recurrences after VATS occur in less than 5% of patients, in which case a redo-VATS is a feasible therapeutical option. Randomized controlled trials are urgently needed to shed light on the best definitive management of SP. Keywords: thoracoscopy; VATS; spontaneous pneumothorax; bullectomy; pleurodesis Citation: Porcel, J.M.; Lee, P. Thoracoscopy for Spontaneous 1. Introduction Pneumothorax. J. Clin. Med. 2021, 10, Pneumothorax can occur spontaneously or because of trauma or procedural compli- 3835. https://doi.org/10.3390/ cation. Spontaneous pneumothoraces (SP) are divided into primary (PSP) and secondary jcm10173835 (SSP). PSP occurs in someone without a known underlying lung disease, whereas SPP appears as a complication of an underlying lung disease, such as chronic obstructive pul- Academic Editors: Paola Ciriaco and Robert Hallifax monary disease, lung cancer, interstitial lung disease, or tuberculosis. -

Coding Billing
CodingCoding&Billing FEBRUARY 2020 Quarterly Editor’s Letter Welcome to the February issue of the ATS Coding and Billing Quarterly. There are several important updates about the final Medicare rules for 2020 that will be important for pulmonary, critical care and sleep providers. Additionally, there is discussion of E/M documentation rules that will be coming in 2021 that practices might need some time to prepare for, and as always, we will answer coding, billing and regulatory compliance questions submitted from ATS members. If you are looking for a more interactive way to learn about the 2020 Medicare final rules, there is a webinar on the ATS website that covers key parts of the Medicare final rules. But before we get to all this important information, I have a request for your help. EDITOR ATS Needs Your Help – Recent Invoices for Bronchoscopes and PFT Lab ALAN L. PLUMMER, MD Spirometers ATS RUC Advisor TheA TS is looking for invoices for recently purchased bronchoscopes and ADVISORY BOARD MEMBERS: PFT lab spirometer. These invoices will be used by theA TS to present practice KEVIN KOVITZ, MD expense cost equipment to CMS to help establish appropriate reimbursement Chair, ATS Clinical Practice Committee rates for physician services using this equipment. KATINA NICOLACAKIS, MD Member, ATS Clinical Practice Committee • Invoices should not include education or service contract as those ATS Alternate RUC Advisorr are overhead and cannot be considered by CMS for this portion of the STEPHEN P. HOFFMANN, MD Member, ATS Clinical Practice Committee formula and payment rates. ATS CPT Advisor • Invoices can be up to five years old. -

Thoracentesis
The new england journal of medicine videos in clinical medicine Thoracentesis Todd W. Thomsen, M.D., Jennifer DeLaPena, M.D., and Gary S. Setnik, M.D. INDICATIONS From the Department of Emergency Medi- Thoracentesis is a valuable diagnostic procedure in a patient with pleural effusion cine, Mount Auburn Hospital, Cambridge, of unknown causation. Analysis of the pleural fluid will allow its categorization as MA (T.W.T., G.S.S.); the Department of Emergency Medicine, Beth Israel Deacon- either a transudate (a product of unbalanced hydrostatic forces) or an exudate (a ess Medical Center, Boston (J.D.); and the product of increased capillary permeability or lymphatic obstruction) (Table 1). If Division of Emergency Medicine, Harvard the effusion seems to have an obvious source (e.g., in an afebrile patient with con- Medical School, Boston (T.W.T., J.D., G.S.S.). Address reprint requests to Dr. Thomsen gestive heart failure and bilateral pleural effusions), diagnostic thoracentesis may at the Department of Emergency Medi- be deferred while the underlying process is treated. The need for the procedure cine, Mount Auburn Hospital, 330 Mount should be reconsidered if there is no appropriate response to therapy.1 Auburn St., Cambridge, MA 02238, or at [email protected]. Thoracentesis, as a therapeutic procedure, may dramatically reduce respiratory distress in patients presenting with large effusions. N Engl J Med 2006;355:e16. Copyright © 2006 Massachusetts Medical Society. CONTRAINDICATIONS There are limited data on the safety of thoracentesis -

Nonintubated Thoracoscopic Surgery Using Regional Anesthesia and Vagal Block and Targeted Sedation
Original Article Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation Ke-Cheng Chen1,2, Ya-Jung Cheng3, Ming-Hui Hung3, Yu-Ding Tseng1, Jin-Shing Chen1,2 1Department of Surgery, National Taiwan University Hospital Yun-Lin Branch, Yun-Lin County, Taiwan; 2Division of Thoracic Surgery, Department of Surgery, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan; 3Department of Anesthesiology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan Corresponding to: Dr. Jin-Shing Chen. Department of Surgery, National Taiwan University Hospital, No. 7, Chung Shan South Road, Taipei, Taiwan. Email: [email protected]. Objective: Thoracoscopic surgery without endotracheal intubation is a novel technique for diagnosis and treatment of thoracic diseases. This study reported the experience of nonintubated thoracoscopic surgery in a tertiary medical center in Taiwan. Methods: From August 2009 through August 2013, 446 consecutive patients with lung or pleural diseases were treated by nonintubated thoracoscopic surgery. Regional anesthesia was achieved by thoracic epidural anesthesia or internal intercostal blockade. Targeted sedation was performed with propofol infusion to achieve a bispectral index value between 40 and 60. The demographic data and clinical outcomes were evaluated by retrospective chart review. Results: Thoracic epidural anesthesia was used in 290 patients (65.0%) while internal intercostal blockade was used in 156 patients (35.0%). The final diagnosis were primary lung cancer in 263 patients (59.0%), metastatic lung cancer in 38 (8.5%), benign lung tumor in 140 (31.4%), and pneumothorax in 5 (1.1%). The median anesthetic induction time was 30 minutes by thoracic epidural anesthesia and was 10 minutes by internal intercostal blockade. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Your Lung Operation Booklet
1 AMERICAN COLLEGE OF SURGEONS DIVISION OF EDUCATION SURGICAL PATIENT EDUCATION Table of Contents Welcome ...................................................................................1 Your Lungs ...............................................................................2 Lung Cancer ...........................................................................3 SURGICAL Understanding Your Operation ........................................4 PATIENT Preoperative Tests ................................................................5 EDUCATION Home Preparation ................................................................8 The Day of Your Operation ...............................................13 After Your Operation ..........................................................14 Your Recovery and Discharge .........................................17 When to Call Your Doctor .................................................19 Welcome You and your family are important members of the surgical team. The American College of Surgeons (ACS) “Your Lung Operation: Education for a Better Recovery” program will help you prepare for your operation and recovery. You and your family will know what to expect. You will learn how to work with your surgical team to ensure that you have the best surgical outcomes. COMPLETE THE “YOUR LUNG OPERATION” EDUCATION PROGRAM: Watch the DVD Read the booklet Review the Medication List and Quit Smoking Resources (inside front cover) Complete the Activity Log (inside front cover) Send us your evaluation after -
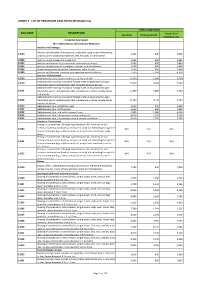
Annex 2. List of Procedure Case Rates (Revision 2.0)
ANNEX 2. LIST OF PROCEDURE CASE RATES (REVISION 2.0) FIRST CASE RATE RVS CODE DESCRIPTION Health Care Case Rate Professional Fee Institution Fee Integumentary System Skin, Subcutaneous and Accessory Structures Incision and Drainage Incision and drainage of abscess (e.g., carbuncle, suppurative hidradenitis, 10060 3,640 840 2,800 cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia) 10080 Incision and drainage of pilonidal cyst 3,640 840 2,800 10120 Incision and removal of foreign body, subcutaneous tissues 3,640 840 2,800 10140 Incision and drainage of hematoma, seroma, or fluid collection 3,640 840 2,800 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst 3,640 840 2,800 10180 Incision and drainage, complex, postoperative wound infection 5,560 1,260 4,300 Excision - Debridement 11000 Debridement of extensive eczematous or infected skin 10,540 5,040 5,500 Debridement including removal of foreign material associated w/ open 11010 10,540 5,040 5,500 fracture(s) and/or dislocation(s); skin and subcutaneous tissues Debridement including removal of foreign material associated w/ open 11011 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 11,980 5,880 6,100 and muscle Debridement including removal of foreign material associated w/ open 11012 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 12,120 6,720 5,400 muscle, and bone 11040 Debridement; skin, partial thickness 3,640 840 2,800 11041 Debridement; skin, full thickness 3,640 840 2,800 11042 Debridement; skin, and -

ANMC Specialty Clinic Services
Cardiology Dermatology Diabetes Endocrinology Ear, Nose and Throat (ENT) Gastroenterology General Medicine General Surgery HIV/Early Intervention Services Infectious Disease Liver Clinic Neurology Neurosurgery/Comprehensive Pain Management Oncology Ophthalmology Orthopedics Orthopedics – Back and Spine Podiatry Pulmonology Rheumatology Urology Cardiology • Cardiology • Adult transthoracic echocardiography • Ambulatory electrocardiology monitor interpretation • Cardioversion, electrical, elective • Central line placement and venous angiography • ECG interpretation, including signal average ECG • Infusion and management of Gp IIb/IIIa agents and thrombolytic agents and antithrombotic agents • Insertion and management of central venous catheters, pulmonary artery catheters, and arterial lines • Insertion and management of automatic implantable cardiac defibrillators • Insertion of permanent pacemaker, including single/dual chamber and biventricular • Interpretation of results of noninvasive testing relevant to arrhythmia diagnoses and treatment • Hemodynamic monitoring with balloon flotation devices • Non-invasive hemodynamic monitoring • Perform history and physical exam • Pericardiocentesis • Placement of temporary transvenous pacemaker • Pacemaker programming/reprogramming and interrogation • Stress echocardiography (exercise and pharmacologic stress) • Tilt table testing • Transcutaneous external pacemaker placement • Transthoracic 2D echocardiography, Doppler, and color flow Dermatology • Chemical face peels • Cryosurgery • Diagnosis -
The Method of Medical Thoracoscopy 2Nd Edition
® THE METHOD OF MEDICAL THORACOSCOPY 2nd Edition Ralf HEINE Jan Hendrik BARTELS Christian WEISS THE METHOD OF MEDICAL THORACOSCOPY 2nd Edition Ralf HEINE, MD Jan Hendrik BARTELS, MD Christian WEISS Medical Clinic III – Pneumonology, Hematology-Oncology and Palliative Medicine Hospital of St. Elisabeth and St. Barbara Halle (Saale), Germany 4 The Method of Medical Thoracoscopy Cover image: The Method of Medical Thoracoscopy Andreas Heine 2nd Edition Ralf Heine, MD Jan Hendrik Bartels, MD Christian Weiss Medical Clinic III – Pneumonology, Hematology-Oncology and Palliative Medicine, Hospital of St. Elisabeth and St. Barbara, Halle (Saale), Germany Correspondence address of the author: Dr. med. Ralf Heine Facharzt für Innere Medizin, Pneumologie Important notes: und Notfallmedizin Medical knowledge is ever changing. As new research and clinical Chefarzt der Medizinischen Klinik III – Pneumologie, experience broaden our knowledge, changes in treat ment and therapy Häma tologie-Onkologie und Palliativmedizin may be required. The authors and editors of the material herein Krankenhaus St. Elisabeth und St. Barbara, Halle/Saale have consulted sources believed to be reliable in their efforts to provide information that is complete and in accord with the Mauerstr. 5 standards accept ed at the time of publication. However, in view of 06110 Halle/Saale, Germany the possibili ty of human error by the authors, editors, or publisher, or changes in medical knowledge, neither the authors, editors, All rights reserved. publisher, nor any other party who has been involved in the prepara- nd | st tion of this booklet, warrants that the information contained herein is 2 edition 1 edition 2007 in every respect accurate or complete, and they are not responsible © 2015 GmbH for any errors or omissions or for the results obtained from use of P.O.