Spatial Distribution of Glossina Sp. and Trypanosoma Sp. in South-Western
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Morphometric Characterization of Three Tsetse Fly Species - Glossina M
International Journal of Environment, Agriculture and Biotechnology (IJEAB) Vol-3, Issue-1, Jan-Feb- 2018 http://dx.doi.org/10.22161/ijeab/3.1.30 ISSN: 2456-1878 Morphometric characterization of three Tsetse Fly Species - Glossina M. Morsitans, G. P. Palpalis and G. Tachinoides (Diptera: Glossinidae) from Ghana Edwin Idriss Mustapha1,2*, Maxwell Kelvin Billah3, Alexander Agyir- Yawson4 1African Regional Postgraduate Programme in Insect Science (ARPPIS), University of Ghana, Legon-Accra, Ghana 2Sierra Leone Agricultural Research Institute, (SLARI) PMB 1313, Tower Hill, Freetown, Sierra Leone 3Animal Biology and Conservation Science, University of Ghana, Box LG 67, Legon-Accra Ghana 4Ghana Atomic Energy Commission, Accra, Ghana Abstract– Tsetse flies (Diptera: Glossinidae) are the main distinguished fly populations into four groups, Northern, vectors of Human African Trypanosomiasis (HAT), or Eastern, Western and the lab colony; this is an indication sleeping sickness and Animal African Trypanosomiasis, that hind tibia/wing length is a good morphometric feature (AAT) or Nagana in Sub Saharan Africa. In Ghana, whilst which can be used to discriminate flies from different HAT is no longer a major public health issue, AAT is still regions of Ghana. widely reported and causes considerable losses in the The principal components and canonical variates as well as livestock sector resulting in major impacts on agricultural Mahalanobis squared distances confirmed linear and ratio production, livelihoods and food security in the country. separations. Therefore based on these differences in Application of morphometric techniques can reveal the morphometric characters observed, the three tsetse species existing level of population differentiation in tsetse flies, were distinguished from each other. -

Study on Spatial Distribution of Tsetse Fly and Prevalence Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Journal of Pharmacy and Alternative Medicine www.iiste.org ISSN 2222-4807 (online) ISSN 2222-5668 (Paper) An International Peer-reviewed Journal Vol.7, 2015 Study on Spatial Distribution of Tsetse Fly and Prevalence of Bovine Trypanosomosis and other Risk Factors: Case Study in Darimu District, Ilu Aba Bora Zone, Western Ethiopia Fedesa Habte Assefa Kebede Tekalegn Desta School of Veterinary Medicine, Jimma University College of Agriculture and Veterinary Medicine, P.O. Box: 307 Jimma, Ethiopia Abstract African Animal Trypanosomosis is one of the major impediments to livestock development and agricultural production in Ethiopia, which negatively affect the overall development in agriculture in general, and to food self- reliance efforts in particular. Currently, about 180,000 to 200,000km 2 of fertile arable land of west and southwest of the country is underutilized. Darimu district is one of the areas with such problems. Therefore, a cross-sectional study was conducted with the objectives of assessing the prevalence of Bovine Trypanosomosis and determines spatial distribution and apparent density of tsetse and other biting flies in the study area. In current study, a total of 650 blood samples were collected from randomly selected animals and subjected to Buffy coat parasitological laboratory technique and positive samples were subjected to thin blood smear followed by Giemsa staining. Out of the total blood sampled, 7.1% tested positive for trypanosomosis. Out of positive cases, Trypanosoma congolense (82.61%) was the dominant trypanosome species followed by mixed infection ( Trypanosoma congolense and Trypanosoma vivax ) (8.67%). -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

Eastern Nile Technical Regional Office
. EASTERN NILE TECHNICAL REGIONAL OFFICE TRANSBOUNDARY ANALYSIS FINAL COUNTRY REPORT ETHIOPIA September 2006 This report was prepared by a consortium comprising Hydrosult Inc (Canada) the lead company, Tecsult (Canada), DHV (The Netherlands) and their Associates Nile Consult (Egypt), Comatex Nilotica (Sudan) and A and T Consulting (Ethiopia) DISCLAIMER The maps in this Report are provided for the convenience of the reader. The designations employed and the presentation of the material in these maps do not imply the expression of any opinion whatsoever on the part of the Eastern Nile Technical Office (ENTRO) concerning the legal or constitutional status of any Administrative Region, State or Governorate, Country, Territory or Sea Area, or concerning the delimitation of any frontier. WATERSHED MANAGEMENT CRA CONTENTS DISCLAIMER ........................................................................................................ 2 LIST OF ACRONYMS AND ABBREVIATIONS .................................................. viii EXECUTIVE SUMMARY ...................................................................................... x 1. BACKGROUND ................................................................................................ 1 1.1 Introduction ............................................................................................. 1 1.2 Primary Objectives of the Watershed Management CRA ....................... 2 1.3 The Scope and Elements of Sustainable Watershed Management ........ 4 1.3.1 Watersheds and River Basins 4 -

Attraction of the Tsetse Fly Glossina Morsitans Submorsitans to Acetone
Retour au menu Rev. Elev. Méd. vét. Pays trop., 1984, 37 (4) : 468-473. Attraction of the tsetse fly ~Glossina morsitans submorsitans to acetone, 1-octen-3-01, and the combination of these compounds in WeSt Africa par H. POLITZAR and P. MÉROT Centre T.E.M.V.T./G.T.Z. de Recherches sur les Trypanosomoses Animales (C.R.T.A.), B. P. 454, Bobo-Dioulasso, Burkina Faso. RÉSUMÉ SUMMARY POLITZAR (H.), MÉROT (P.). - Pouvoir attractif pour POLITZAR (H.), MÉROT (P.). - Attraction of the Glossina morsitans submorsitans de l’acétone, du l-octen- tsetse fly Glossina morsitans submorsitans to acetone, 3-01 seuls ou associés, en Afrique occidentale. Rev. Eh. 1-octen-3-01, and the combination of these compounds in Méd. vét. Pays trop., 1984, 37 (4) : 468-413. West Africa. Rev. Elev. Méd vét. Pqs trop., 1984, 31 (4) : 468-473. Le pouvoir attractif du 1-octen-3-01 (octenol) et de I’acé- tone ayant été montré au Zimbabwé pour G. pallidipes et 1-octen-3-01 (= octenol) and acetone that had proved to G. m. morsitans, ces deux produits ont été testés vis-à-vis be potent olfactory attractants in Zimbabwe for G. palli- de G. m. submorsitans au Burkina Faso. Les essais, faits dipes and G. m. morsitans were tested against G. m. successivement en saison des pluies puis en saison sèche, submorsitans in Burkina Faso. Experiments were carried ont été réalisés selon le protocole des carrés latins. out in the rainy season and in the dry season. TO compare L’analyse des résultats obtenus en saison des pluies a mis the efficacy of acetone, octenol, acetone-plus-octenol - en évidence un accroissement significatif des captures de baited and non baited traps, a series of randomised 4 x 4 6,7 fois lorsque les deux produits étaient associés au piège. -
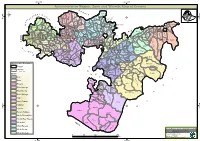
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

The Distribution of the Vectors of African Pathogenic Trypanosomes*
Bull. Org. mond. Sante 1963, 2.8, 653-669 Bull. Wld Hth Org. I The Distribution of the Vectors of African Pathogenic Trypanosomes* J. FORD 1 The author lists the species, subspecies and races of tsetse fly now recognized in three morphologically distinct groups. The distribution of each group is mapped and described in relation to climate and vegetation, with some indication ofthe partplayed bypast climates and orogenies in determining the modern pattern. The importance of different species as vectors of human or bovine trypanosomiasis, or both, is noted, and examples are given of the part played by human settlement as a secondary limiting factor. The author suggests that many modern problems of control are the consequences of the recent invasion of the African ecosystem by the outside world. Although there are local exceptions, the broad pattern of Glossina distribution has not been significantly changed by the entomological approach to the trypanosomiasis problem. So little is known of the role of haematophagous belt of the Koalib Hills in Kordofan and at least insects other than tsetse flies as vectors of African six species in scattered foci in western and southern pathogenic trypanosomes that, although the so- Ethiopia. These relics have survived a southward called " mechanical transmission" occasionally expansion of the Sahara (see, for example, Huzayyin, assumes local importance, discussion must be con- 1956). fined to Glossina (Muscidae, Diptera). The genus Bursell (1960) has suggested that climates showing occurs only in Africa, between latitude 14°N and a mean temperatures for the length of a pupal period line drawn from Benguela to Durban which is of 16°C or less (three months) or of 32°C (three indented by the Bihe Plateau in Angola (Sousa Dias, weeks) provide limits to the distribution of G. -

Original Article
Available online at http://www.journalijdr.com ISSN: 2230-9926 International Journal of Development Research Vol. 08, Issue, 01, pp.18459-18464, January, 2018 ORIGINAL RESEARCH ARTICLEORIGINAL RESEARCH ARTICLE OPEN ACCESS STUDY OF DISTRIBUTION AND ABUNDANCE OF TSETSE FLY (GLOSSINA SP.) IN GASHAKA-GUMTI NATIONAL PARK, NIGERIA 1,*Wama B. E., 2Naphtali, R.S., 1Houmsou, R.S., 1Joseph J., 2Alo, E. B. 1Department of Biological Sciences, Taraba State University, Jalingo, Nigeria 2Department of Zoology, Modibbo Adama University of Technology Yola, Adamawa State, Nigeria ARTICLE INFO ABSTRACT Article History: A study on the distribution and abundance of tsetse flies was conducted between November, 2016 Received 25th October, 2017 and January, 2017 at Gashaka-Gumti National Park (GGNP), Nigeria. The aim of the study was to Received in revised form determine the distribution and abundance of the fly (Glossina sp.) in the Park. Thirty (30) 06th November, 2017 Biconical traps (Charlier and Laviessiere, 1973) were used to trap the tsetse flie in three locations Accepted 20th December, 2017 (Kwano, Gashaka and Mayo-kam). A total of six hundredand ninety eight (698) flies were caught st Published online 31 January, 2018 during the study period. Kwano, Gashaka and Mayo-kam had 372 (53.3%), 168 (24.1%) and 158 (22.6%) respectively. Location of the traps varied significantly with tsetse catch (χ2 = 250.150; Key Words: P<0.000). Glossina tachinoides. Glossina palpalis, Glossina morsitans and Glossina fuscipes were Distribution, trapped in the area. Overall, Glossina tachinoides had higher frequency of 476 (68.2%) and least Abundance, from Glossina fuscipes 6 (0.9%). -

The Role of Indigenous Healing Practices in Environmental Protection Among the Maccaa Oromo of Ilu Abbaa Bora and Jimma Zones, Ethiopia
Available online at www.sserr.ro Social Sciences and Education Research Review (4) 1 30-53 (2017) ISSN 2393–1264 ISSN–L 2392–9863 THE ROLE OF INDIGENOUS HEALING PRACTICES IN ENVIRONMENTAL PROTECTION AMONG THE MACCAA OROMO OF ILU ABBAA BORA AND JIMMA ZONES, ETHIOPIA Milkessa Edae TUFA1 , Fesseha Mulu GEBREMARIAM2 1Department of Oromo Folklore and Literature, Jimma University, Ethiopia E-mail: [email protected] 2Department of Governance and Development Studies, Jimma University, Ethiopia E-mails: [email protected] or [email protected] Abstract This article mainly attempted to explore the role of utilizing indigenous medicines in environmental protection among the Maccaa Oromo of Jimma and Iluu Abba bora zone, south-western Ethiopia. To this end, 4 separate interviews with 4 interviewees, 2 focus group discussions with 17 participants, and non- participant field observation were conducted to generate significant and reliable data. Besides, the researchers employed secondary data to make the study more significant and complete. The findings of the study show that since the source of medicines is the environment, the community protects their environment unless the society wouldn’t accessed the natural medicines they need. The study also reveals that most of these folk medicines used by the Maccaa Oromos are from 30 plants. This further indicates the society protects the natural environment to get the plants they use for medication. Thus, folk healing practices are crucial on the one hand to treat illnesses, and to protect the ecosystem on the other hand. However, these societal knowledge is undermined as well as they are being replaced by western (scientific) knowledge, modern medicines. -

The Glossina Genome Cluster: Comparative Genomic Analysis of the Vectors of African
bioRxiv preprint doi: https://doi.org/10.1101/531749; this version posted January 27, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The Glossina Genome Cluster: Comparative Genomic Analysis of the Vectors of African 2 Trypanosomes 3 Authorship: 4 Geoffrey M. Attardo, ([email protected]) *22; Adly M.M. Abd-Alla, (a.m.m.abd- 5 [email protected])13; Alvaro Acosta-Serrano, ([email protected])16; James E. 6 Allen, ([email protected])6; Rosemary Bateta, ([email protected])2; Joshua B. Benoit, 7 ([email protected])24; Kostas Bourtzis, ([email protected])13; Jelle Caers, 8 ([email protected])15; Guy Caljon, ([email protected])21; Mikkel B. Christensen, 9 ([email protected])6; David W. Farrow, ([email protected])24; Markus Friedrich, 10 ([email protected])33; Aurélie Hua-Van, ([email protected])5; Emily C. 11 Jennings, ([email protected])24; Denis M. Larkin, ([email protected])19; Daniel Lawson, 12 ([email protected])10; Michael J. Lehane, ([email protected])16; Vasileios 13 P. Lenis, ([email protected])30; Ernesto Lowy-Gallego, ([email protected])6; 14 Rosaline W. Macharia, ([email protected], [email protected])27,12; Anna R. Malacrida, 15 ([email protected])29; Heather G. Marco, ([email protected])23; Daniel Masiga, 16 ([email protected])12; Gareth L. Maslen, ([email protected])6; Irina Matetovici, 17 ([email protected])11; Richard P.