Potential of Membrane Technology for Treatment and Reuse of Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pulih Sepenuhnya Pada 8:00 Pagi, 21 Oktober 2020 Kumpulan 2
LAMPIRAN A SENARAI KAWASAN MENGIKUT JADUAL PELAN PEMULIHAN BEKALAN AIR DI WILAYAH PETALING, GOMBAK, KLANG/SHAH ALAM, KUALA LUMPUR, HULU SELANGOR, KUALA LANGAT DAN KUALA SELANGOR 19 OKTOBER 2020 WILAYAH : PETALING ANGGARAN PEMULIHAN KAWASAN Kumpulan 1: Kumpulan 2: Kumpulan 3: Pulih Pulih Pulih BIL. KAWASAN sepenuhnya sepenuhnya sepenuhnya pada pada pada 8:00 pagi, 8:00 pagi, 8:00 pagi, 21 Oktober 2020 22 Oktober 2020 23 Oktober 2020 1 Aman Putri U17 / 2 Aman Suria / 3 Angkasapuri / 4 Bandar Baru Sg Buloh Fasa 3 / 5 Bandar Baru Sg. Buloh Fasa 1&2 / 6 Bandar Baru Sri Petaling / 7 Bandar Kinrara / 8 Bandar Pinggiran Subang U5 / 9 Bandar Puchong Jaya / 10 Bandar Tasek Selatan / 11 Bandar Utama / 12 Bangsar South / 13 Bukit Indah Utama / 14 Bukit Jalil / 15 Bukit Jalil Resort / 16 Bukit Lagong / 17 Bukit OUG / 18 Bukit Rahman Putra / 19 Bukit Saujana / 20 Damansara Damai (PJU10/1) / 21 Damansara Idaman / 22 Damansara Lagenda / 23 Damansara Perdana (Raflessia Residency) / 24 Denai Alam / 25 Desa Bukit Indah / 26 Desa Moccis / 27 Desa Petaling / 28 Eastin Hotel / 29 Elmina / 30 Gasing Indah / 31 Glenmarie / 32 Hentian Rehat dan Rawat PLUS (R&R) / 33 Hicom Glenmarie / LAMPIRAN A SENARAI KAWASAN MENGIKUT JADUAL PELAN PEMULIHAN BEKALAN AIR DI WILAYAH PETALING, GOMBAK, KLANG/SHAH ALAM, KUALA LUMPUR, HULU SELANGOR, KUALA LANGAT DAN KUALA SELANGOR 19 OKTOBER 2020 WILAYAH : PETALING ANGGARAN PEMULIHAN KAWASAN Kumpulan 1: Kumpulan 2: Kumpulan 3: Pulih Pulih Pulih BIL. KAWASAN sepenuhnya sepenuhnya sepenuhnya pada pada pada 8:00 pagi, 8:00 pagi, 8:00 -

Puchong (North) BUDGET 2019 EDITION
BUDGET 2019 EDITION by Henry Butcher Malaysia BUDGET 2019 AND ITS IMPACT ON THE PROPERTY INDUSTRY IN MALAYSIA PLUS When The Going Gets Tough, Selangor: Dip in New Value Map Series: KDN PP18893/11/2015(034373) Seek Wisdom Launches in H1 2018 Puchong (North) BUDGET 2019 EDITION by Henry Butcher Malaysia BUDGET 2019 AND ITS IMPACT ON THE PROPERTY INDUSTRY IN MALAYSIA PLUS The Pursuit Of Opulence With Selangor New Launches 8alue Map Series: KDN PP18893/11/2015(034373) Ritz Carlton Residences, KL H1 2017 vs 2018 Puchong (North) BUDGET 2019 EDITION Editor’s Note by Henry Butcher Malaysia Publisher Henry Butcher Malaysia Sdn Bhd 25, Jalan Yap Ah Shak, O Jalan Dang Wangi, 50300 Kuala Lumpur. T• (03) 2694 2212 E• [email protected] W• www.henrybutcher.com.my OUR SERVICES Valuation I recently attended a seminar conducted by take-up rates for those developments Tel :603-26942212 Fax: 603-26943484 Lembaga Perumahan dan Hartanah which are not conducive for the lower Email: [email protected] Selangor (Housing and Property Board, income households, the issue of mainte- Project Marketing Selangor) on the launch of their latest nance charge collection, the issue of Tel: 603-26942212 Fax: 603-26925771 policy guidelines on “aordable housing “ amenities and facilities for the open Email: [email protected] in Selangor titled “Dasar Perumahan dan market household and the “aordable” Real Estate Agency Hartanah Mampu Milik Selangor 2.0”. household. Tel: 603-26942212 Fax: 603-26941261 Aer 4 years in existence since January Email: [email protected] 2014, this is an update of the previous Malaysia is probably one of the few Market Research & Development Consultancy housing policy of the State known as countries in the world that have shied the Tel: 603-42702072 Fax: 603-42702082 “Rumah Selangorku”. -

Senarai Utama Klinik Panel Upm
SENARAI UTAMA KLINIK PANEL UPM TEMPOH LANTIKAN: 01 JANUARI 2017 - 31 DISEMBER 2020 SERI KEMBANGAN NAMA KLINIK ALAMAT TEL/FAKS 1 Poliklinik Penawar 3344, Jln 18/32 03-89481991 24 Jam Tmn Sri Serdang 43300 Seri Kembangan, 03-89456991 Selangor 2 Pusat Rawatan Mesra 26, Jln 7/2, Sri Serdang 03-89459485 8:30 Pagi - 10:00 Malam 43300 Seri Kembangan, Selangor - sama - 3 Klinik R. Daya No.9, Jln Desa Serdang 1 03-89456976 9:00 Pagi – 9:00 Malam Tmn Desa Serdang 43300 Serdang, Selangor - sama - 4 Klinik Tg. Mohd & Rakan- 3258, Jln 18/37 03-89482045 Rakan Tmn Sri Serdang 8:00 Pagi - 10:00 Malam 43300 Seri Kembangan, 03-89424697 Selangor 5 Klinik Fadzliyana 63, Jln LP 1A/2 03-89436160 8:30 Pagi - 10:00 Malam Tmn Lestari Perdana 43300 Seri Kembangan, 03-89435350 Selangor 6 Klinik Mani & Surgeri 3268, Jln 18/36 03-89486190 8:00 Pagi - 10:00 Malam Taman Sri Serdang 43300 Seri Kembangan, 03-89425601 Selangor 7 Klinik Qistina No. 27-1, Jalan BPP 5/1 03-89434487 8:00 Pagi - 10:00 Malam Permai Square 1 012-9448246 Bandar Putra Permai 43300 Seri Kembangan Selangor 03-89434487 8 Clinic Mediviron 15G, Jln LP 2A/1 03-89422079 24 Jam Taman Lestari Perdana 43300 Seri Kembangan - sama - Selangor 9 Poliklinik An Nur (Bandar 03-89455431 No.15 BPP 8/1, Pusat Bandar Putra Permai) Putra Permai, 43300 Seri - sama - Kembangan, Selangor 10 Poliklinik An Nisa (Bandar No. 3 Jalan Bandar Putra 03-89458414 Putra Permai) Permai 8/1 Bandar Putra Permai, - sama - 43300 Seri Kembangan Selangor 11 Klinik Mediviron Serdang No 24, Jalan Raya 7/2 03 89381009 Jaya Taman Serdang Jaya 03 89381009 43300 Seri Kembangan Selangor 12 Klinik Shukor G -56, Jalan SP 5/4 03 89581898 Taman Serdang Perdana 03 89581898 43300 Seri Kembangan Selangor 13 Klinik Mega 39 Jalan Putra Permai 1A, 03 89413969 Equine Park 03 89413969 43300 Seri Kembangan Selangor BALAKONG NAMA KLINIK ALAMAT TEL/FAKS 14 Klinik Selva No. -

Senarai Semua Lokasi Hotspot Wifi Smart Selangor Adalah Seperti Berikut
Senarai semua lokasi hotspot WiFi Smart Selangor adalah seperti berikut:- No. Site Address Category 1 Masjid Nurul Yaqin Mosque Kampung Melayu Seri Kundang, 48050 Rawang, Selangor 2 Pusat Gerakan Khidmat Masyarakat (DUN Kuang) Government 6-1-A, Jalan 7A/2, Bandar Tasik Puteri, 48000 Rawang, Selangor 3 HOSPITAL SUNGAI BULOH_300014, 47000 Hospital Sungai Buloh Selangor 4 HOSPITAL SUNGAI BULOH_300014 Hospital 5 HOSPITAL SUNGAI BULOH_300014 Hospital 6 HOSPITAL SUNGAI BULOH_300014 Hospital 7 HOSPITAL SUNGAI BULOH_300014 Hospital 8 HOSPITAL SUNGAI BULOH_300014 Hospital 9 Perodua Service Centre Jln Sungai Pintas, No.14, Commercial Jalan TSB 10, Taman Industri Sg. Buloh 47000 Shah Alam selangor 10 TESCO RAWANG_300026, No.1, Jalan Rawang Mall 48000 Rawang Selangor 11 TM POINT RAWANG, TM Premises Lot 21, Jalan Maxwell 48000 Rawang 12 Stadium MPS, Jalan Persiaran 1, Bandar Baru Stadium Selayang, 68100 Batu Caves, Selangor 13 Pejabat Cawangan Rawang, Jalan Bandar Rawang Government 2, Bandar Baru Rawang, 48000 Rawang, Selangor 14 No. 309 Felda Sungai Buaya, 48010 Rawang, Residential Selangor area 15 Traffic Light Chicken Rice Sungai Choh, 48009 F&B outlet Rawang, Selangor 16 Pejabat Khidmat Rakyat (DUN Rawang) Government No.13, Jalan Bersatu 8 (Tingkat Bawah), Taman Bersatu, 48000 Rawang, Selangor 17 WTC Restoran F&B Outlet Rawang new town, 48000 Rawang, Selangor 18 Medan Selera MPS F&B Outlet Rawang Integrated Industrial Park, 45000 Rawang, Taman Tun Teja, Rawang, Selangor 19 Medan Selera F&B Outlet Bandar Country Homes, 48000 Rawang, Selangor 20 Kompleks JKKK, Selayang Baru, JKR 750C, Dewan Government Orang Ramai, Jalan Besar Selayang Baru, 68100 Batu Caves, Selangor 21 Pejabat Ahli Parlimen Selayang,12A, Jalan SJ 17, Government Taman Selayang Jaya, 68100 Batu Caves, Selangor No. -

No. POS Outlet Address Contact 1 Tanjong Sepat JKR 4609 A-B
No. POS Outlet Address Contact JKR 4609 A-B, Jalan Besar, 1 Tanjong Sepat 03-31974260 42800, Tanjong Sepat JKR 1348, Jln Besar, 42500, 2 Telok Panglima Garang 03-31227755 Telok Panglima Garang JKR 1043, Jalan Sungai 3 Jenjarom 03-31913221 Buaya, 46200, Jenjarom Lot No. 16, Jalan Berjuntai 3, Pusat Perniagaan Batang 4 Batang Berjuntai 03-32719161 Berjuntai, 45650, Batang Berjuntai No. 74, Jalan Jati, 45800, 5 Jeram 03-32647171 Jeram No. 3, Rantau Panjang, 6 Rantau Panjang 03-32912591 42100, Rantau Panjang JKR 143, Jln Besar, 44300, 7 Batang Kali 03-60573232 Batang Kali No. 40-G-A, Jalan 7A/2, 8 Bandar Tasik Puteri Bandar Tasik Puteri, 48020, 03-60914706 Rawang No. 1, Jalan BP 7/13 Mukim 9 Bandar Bukit Puchong Rawang, Bandar Bukit 03-80633744 Puchong, 47100, Puchong Lot 148/149, Tkt. Bawah Tesco Stores (M) Sdn Bhd 10 Tesco Puchong Jaya 03-80768744 Jln Bandar Puchong, 47100, Puchong Lot F60, 1st Floor, AEON 11 Jusco Bukit Tinggi Bukit Tinggi Shopping 03-33262796 Centre, 41200, Klang JKR 387, Jalan Ulu Langat, 12 Ulu Langat 03-90211314 43100, Ulu Langat SSK 6 - SSR 7 Hentian 13 Bukit Dukung Rehat & Rawat, Bukit 03-87395113 Dukong, 43000, Kajang Lot F27, KMB Aeon Cheras 14 Jusco Cheras Selatan Selatan, Lebuh Tun Hussein 03-90742914 Onn, 43200, Balakong JKR 208 A-B, Jalan 6C/9, 15 Bandar Baru Bangi 03-89259555 43000, Bandar Baru Bangi Universiti Kebangsaan Bangunan Pusanika UKM, 16 03-89250551 Malaysia Bangi, , 43600, Bangi Lot F24A, Tingkat 1, Jusco 17 Jusco Bukit Raja 03-33429396 Bukit Raja, 41150, Klang Pasar Besar Klang, Klang, , 18 Pasar Besar Klang 03-33440210 41052, Klang Lot S.11, 2nd Floor Selayang Mall, Jalan SU 9 19 Selayang Mall 03-61370533 Taman Selayang Utama, 68100, Batu Caves Lot LL 3.03 Sunway Pyramid, No. -
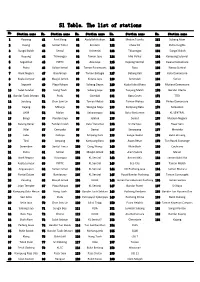
S1 Table. the List of Stations
S1 Table. The list of stations No Station name No. Station name No. Station name No. Station name No. Station name . 1 Rawang 41 Port Klang 81 Abdullah Hukum 121 Medan Tuanku 161 Subang Alam 2 Kuang 42 Sentul Timur 82 Kerinchi 122 Chow Kit 162 Putra Heights 3 Sungai Buloh 43 Sentul 83 Universiti 123 Titiwangsa 163 Sungai Buloh 4 Kepong 44 Titiwangsa 84 Taman Jaya 124 Mid Valley 164 Kampung Selamat 5 Segambut 45 PWTC 85 Asia Jaya 125 Kepong Sentral 165 Kwasa Damansara 6 Putra 46 Sultan Ismail 86 Taman Paramount 126 Rasa 166 Kwasa Sentral 7 Bank Negara 47 Bandaraya 87 Taman Bahagia 127 Batang Kali 167 Kota Damansara 8 Kuala Lumpur 48 Masjid Jamek 88 Kelana Jaya 128 Serendah 168 Surian 9 Seputeh 49 Plaza Rakyat 89 Subang Depot 129 Kuala Kubu Bharu 169 Mutiara Damansara 10 Salak Selatan 50 Hang Tuah 90 Subang Jaya 130 Tanjung Malim 170 Bandar Utama 11 Bandar Tasik Selatan 51 Pudu 91 Gombak 131 Batu Caves 171 TTDI 12 Serdang 52 Chan Sow Lin 92 Taman Melati 132 Taman Wahyu 172 Phileo Damansara 13 Kajang 53 Miharja 93 Wangsa Maju 133 Kampung Batu 173 Semantan 14 UKM 54 Maluri 94 Setiawangsa 134 Batu Kentomen 174 KL SENTRAL 15 Bangi 55 Pandan Jaya 95 Jelatek 135 Sentul 175 Muzium Negara 16 Batang Benar 56 Pandan Indah 96 Dato' Keramat 136 Sri Rampai 176 Pasar Seni 17 Nilai 57 Cempaka 97 Damai 137 Senawang 177 Merdeka 18 Labu 58 Cahaya 98 Ampang Park 138 Sungai Gadut 178 Bukit Bintang 19 Tiroi 59 Ampang 99 Kampung Baru 139 Awan Besar 179 Tun Razak Exchange 20 Seremban 60 Sentul Timur 100 Dang Wangi 140 Muhibbah 180 Cochrane 21 Putra 61 Sentul -

DEVI KAVITHA A/P KRISHNA Sek
DEVI KAVITHA A/P KRISHNA Sek. Keb. Puchong Perdana Jalan Perdana 4/2, Tmn. Puchong Perdana, 47100 Selangor, Malaysia. +6016 3778846 [email protected] Career Focus . To use my skills and contribute for the profits my employer. To conquer the challenges faced as an educator and a freelance translator. Experience Summary Well versed in copyediting, translating, and proofreading, most recently assisted with a few proposals and books for the Sek Keb Puchong Perdana (Primary School). Dedicated primary teacher been teaching student/pupils from different groups and background for the past 8 years. Strong technical command of the English language and Bahasa Malaysia (Malay) Competent researcher, educator and examiner. Education 2010 – BACHELORS DEGREE WITH HONOURS IN ENGLISH currently STUDIES (2014) Malaysian Open University, Petaling Jaya, Selangor, Malaysia. 2003-2005 DIPLOMA IN TEACHING OF ENGLISH LANGUAGE Perlis Teachers Training Institution, Malaysia. Professional 2009- TEACHER Currently Experience SEK. KEB.PUCHONG PERDANA 2006-2008 TEACHER SJK(T) DESA CEMPAKA, NILAI 2005-2006 PRACTICAL TEACHER SK DATO AHMAD SAID,PERLIS TEACHER (Part Time) 2009 - Currently EZ2SCORE ISIAN MINDA, PUCHONG TEACHER (Part Time) 2011-currently SINAR CERDIK, PUTRA PRIMA PUCHONG LITIGATION CLERK 2000-2002 KHALED, MUTANG, CHAN AND LIM, KUALA LUMPUR Computer Skills • Microsoft Word • Microsoft Excel • Microsoft PowerPoint Skills . Proofreading . Planning and Teaching English as Second Language . Editing . Setting exam papers . Marking exam papers . Translating notes and exercise . Classroom discipline books . Analyzing exam papers LANGUAGE SKILLS: SPOKEN WRITTEN READ MALAY Excellent Excellent Excellent ENGLISH Excellent Excellent Excellent TAMIL Good Hardly Hardly References Available. . -

Download File
Cooperation Agency Japan International Japan International Cooperation Agency SeDAR Malaysia -Japan About this Publication: This publication was developed by a group of individuals from the International Institute of Disaster Science (IRIDeS) at Tohoku University, Japan; Universiti Teknologi Malaysia (UTM) Kuala Lumpur and Johor Bahru; and the Selangor Disaster Management Unit (SDMU), Selangor State Government, Malaysia with support from the Japan International Cooperation Agency (JICA). The disaster risk identification and analysis case studies were developed by members of the academia from the above-mentioned universities. This publication is not the official voice of any organization and countries. The analysis presented in this publication is of the authors of each case study. Team members: Dr. Takako Izumi (IRIDeS, Tohoku University), Dr. Shohei Matsuura (JICA Expert), Mr. Ahmad Fairuz Mohd Yusof (Selangor Disaster Management Unit), Dr. Khamarrul Azahari Razak (Universiti Teknologi Malaysia Kuala Lumpur), Dr. Shuji Moriguchi (IRIDeS, Tohoku University), Dr. Shuichi Kure (Toyama Prefectural University), Ir. Dr. Mohamad Hidayat Jamal (Universiti Teknologi Malaysia), Dr. Faizah Che Ros (Universiti Teknologi Malaysia Kuala Lumpur), Ms. Eriko Motoyama (KL IRIDeS Office), and Mr. Luqman Md Supar (KL IRIDeS Office). How to refer this publication: Please refer to this publication as follows: Izumi, T., Matsuura, S., Mohd Yusof, A.F., Razak, K.A., Moriguchi, S., Kure, S., Jamal, M.H., Motoyama, E., Supar, L.M. Disaster Risk Report by IRIDeS, Japan; Universiti Teknologi Malaysia; Selangor Disaster Management Unit, Selangor State Government, Malaysia, 108 pages. August 2019 This work is licensed under a Creative Commons Attribution-Non Commercial-Share Alike 4.0 International License www.jppsedar.net.my i Acknowledgement of Contributors The Project Team wishes to thank the contributors to this report, without whose cooperation and spirit of teamwork the publication would not have been possible. -

For Sale - Taman Putra Prima Puchong Perdana Bukit Puchong, Puchong, Selangor
iProperty.com Malaysia Sdn Bhd Level 35, The Gardens South Tower, Mid Valley City, Lingkaran Syed Putra, 59200 Kuala Lumpur Tel: +603 6419 5166 | Fax: +603 6419 5167 For Sale - Taman Putra Prima Puchong Perdana Bukit Puchong, Puchong, Selangor Reference No: 102471662 Tenure: Freehold Address: Jalan PP5 Taman Putra Prima, Unit Type: Intermediate Taman Putra Prima Puchong Land Title: Residential Perdana Bukit Puchong, Selangor Property Title Type: Individual State: Selangor Posted Date: 10/09/2021 Property Type: 2-sty Terrace/Link House Asking Price: RM 755,000 Built-up Size: 2,200 Square Feet Built-up Price: RM 343.18 per Square Feet Land Area Size: 1,650 Square Feet Land Area Price: RM 457.58 per Square Feet No. of Bedrooms: 4 No. of Bathrooms: 3 Name: Nick Sim Company: POLYGON PROPERTIES SDN BHD Email: [email protected] Taman Putra Prima 2 storey House For Sale RM755k ONLY!!!!! Taman Putra Prima Jalan PP 5 Puchong Perdana 2 storey House For Sale!!!!! - LA 20x70sf - BU 2200sf - Freehold Amenities : - MASA College - Binary University College - Monash University Malaysia -Taylor University Lakeside Campus - IOI Mall Puchong - Tesco Puchong - NSK Bandar Puteri - Carrefour Puchong - Columbia Asia Medical Centre - Sunway Medical Centre Accessibility : - LDP - ELITE - KESAS - NPE - KIDEX - SKIP Sri Petaling Bukit Jalil OUG Kuchai Lama Old Klang Road Puchong Taman Equine Seri Kembangan Kajang Bangi Setia.... [More] View More Details On iProperty.com iProperty.com Malaysia Sdn Bhd Level 35, The Gardens South Tower, Mid Valley City, Lingkaran Syed Putra, 59200 Kuala Lumpur Tel: +603 6419 5166 | Fax: +603 6419 5167 For Sale - Taman Putra Prima Puchong Perdana Bukit Puchong, Puchong, Selangor. -

Kemaskini 18 Julai 2020 Bil Dun Terkini Bandar/Pekan/Taman Nama Kedai Alamat Telefon Tarikh Mid 1 N01 Sg. Air Tawar Pekan Simpan
KEMASKINI 18 JULAI 2020 SENARAI PANEL KEDAI PROGRAM KASIH IBU SMART SELANGOR (KISS) BIL DUN TERKINI BANDAR/PEKAN/TAMAN NAMA KEDAI ALAMAT TELEFON TARIKH MID 1 N01 SG. AIR TAWAR PEKAN SIMPANG EMPAT 96 MINI MARKET PEKAN SIMPANG 4 BNO, 45200, SABAK BERNAM, SELANGOR DARUL EHSAN. 016-2843617 18 January 2018 80002998 2 N01 SG. AIR TAWAR KAMPUNG BAHARU TARAMERAJU S/O KALLY KAMPUNG BAHARU, N0. 41, 45200, SABAK BERNAM, SELANGOR DARUL EHSAN. 019-6448243 19 January 2018 80003038 3 N01 SG. AIR TAWAR JALAN PASAR 96 MINI MARKET (SG. AYER TAWAR) NO. 23, JALAN PASAR, 45100 SG. AYER TAWAR, SELANGOR DARUL EHSAN. 016-2843617 19 January 2018 80002994 4 N01 SG. AIR TAWAR BATU 37 SITAZ TRADING & SERVICES BATU 37, SIMPANG 4, 019-3364941 22 January 2018 80002997 5 N01 SG. AIR TAWAR JALAN RAJA CHULAN PERNIAGAAN RUNCIT M.MOHD.ABDUL KADERNO. 33 JLN RAJA CHULAN, 45200 SABAK BERNAM, SELANGOR DARUL EHSAN. 019-3241735 23 April 2018 80003030 6 N01 SG. AIR TAWAR KG DATO HORMAT ASMALI JAYA ENTERPRISE LOT 5440, KG. DATO HORMAT, SG. NIREH, 45100, SG. AIR TAWAR, SELANGOR DARUL EHSAN. 019-6195063 18 January 2018 80003041 7 N01 SG. AIR TAWAR JALAN PEKAN PARIT BARU FASHA FROZEN LOT 430, N0 82, JALAN PEKAN PARIT BARU, 45100, SG. AIR TAWAR, SELANGOR DARUL EHSAN. 017-2234429 18 January 2018 80002993 LOT 619 (PT 1799) & 620 (PT 1800) GF, JALAN RAJA CHULAN, TAMAN MUTIARA 2 , SEKSYEN 5, PEKAN SABAK BERNAM, 45200 SABAK BERNAM, SELANGOR 8 N02 SABAK PEKAN SABAK BERNAM 99 SPEEDMART N/A 15 February 2018 80003402 DARUL EHSAN. -

LAMPIRAN a KLANG Aman Perdana Jalan Samarinda Perumahan Bandar Sultan Sulaiman Fasa 3 Ambang Botanik Jalan Stesen & Solok B
LAMPIRAN A KLANG Perumahan Bandar Sultan Aman Perdana Jalan Samarinda Sulaiman Fasa 3 Ambang Botanik Jalan Stesen & Solok Besar PKFZ Armada Putera Jalan Syahbandar Pulau Ketam Bandar Baru Bukit Raja Jalan Tengku Badar Sementa Sepanjang Jalan Goh Hock Bandar Bestari Jalan Tengku Diauddin Huat Bandar Botanik Jalan Tengku Kelana Sungai Bertik Bandar Bukit Tinggi 1 Jalan Tepi Sungai Sungai Kapar Indah Bandar Bukit Tinggi 2 Jalan Yadi Sungai Puloh Bandar Klang (Bt 1 - 3 Jalan Johan Setia Sungai Putus Meru) Bandar Parkland Kampung Budiman Sungai Sireh Bandar Putera Kampung Bukit Cerakah Sungai Udang Bandar Putera 2 Kampung Delek Taman Bayu Mas Kampung Jawa, Kampung Bandar Puteri Taman Bayu Perdana Jawa Batu Belah dan Teluk Kapas Kampung Kastam Taman Bayu Tinggi Berkeley/Eng Ann Kampung Keretapi Taman Berembang Taman Bijaya Kampung Bukit Kapar Kampung Pendamar Jawa Bukit Kerayong Kampung Pulau Indah Taman Chi Liung Bukit Kuda & Jalan Batu 3 Kampung Raja Uda Taman Desawan Lama Felda Bukit Cerakah Kampung Teluk Gong Taman Gembira Glenmarie Cove Kapar Taman Klang Jaya Kaw. Perindustrian Bandar Glenn Cruise Sultan Sulaiman dan Taman Maznah Perdana Ind. Park Kawasan Perindustrian Bukit Hospital Ampuan Rahimah Taman Palm Groove Raja Selatan Jalan Dato' Hamzah Klang Sentral Taman Pendamar Indah Jalan Istana Kota Pendamar Taman Petaling Indah Jalan Kapar Bt 1 - 8 & Klang Taman Radzi Teluk Gadong Laguna Park Utama Besar Jalan Kem Lorong Tingkat & Rembau Taman Samudera Jalan Klang Banting Meru Taman Selatan Jalan Kota Pandamaran Taman Sentosa Jalan Kota -

Contents Company Overview the Firm Our Services Organisation Chart Our People & Strength Certificate & Recognition Projects
CONTENTS COMPANY OVERVIEW PAGE 1 THE FIRM PAGE 2 OUR SERVICES PAGE 3 ORGANISATION CHART PAGE 5 OUR PEOPLE & STRENGTH PAGE 6 CERTIFICATE & RECOGNITION PAGE 9 PROJECTS PAGE 19 ENVIRONMENTAL IMPACT ASSESSMENT (EIA) MIXED DEVELOPMENT WITH STP EIA ENVIRONMENTAL MANAGEMENT PLAN (EMP) ENVIRONMENTAL MONITORING REPORT (EMR) ENVIRONMENTAL COMPLIANCE PROGRAM (ECP) POST-EIA STUDIES OTHER STUDIES COMPANY OVERVIEW EUROPASIA ENGINEERING SERVICES SDN BHD is a local environmental consultancy registered with the Department of Environment (F00054) and is one of the leading consultants in EIA and Post EIA work. Incorporated in 1992, EUROPASIA has to-date secured approval for more than 200 environmental related studies and has achieved an impressive success track record. EUROPASIA has also obtained ISO 9001:2015 Certification for Environmental Consultancy and Training Services in its efforts to continuously upgrade its services. EUROPASIA offers services in the field of Environmental Impact Assessments (EIA); preliminary and detailed, environmental audits, Post EIA compliance reporting and environmental studies. Employing multi-disciplinary personnel with the required certification to undertake EIAs, EUROPASIA is able to be a one-stop consultancy to undertake studies and audits. EUROPASIA strongly believes that through offering a multitude of services in the environmental sector, it would result in a positive long term effect on the deteriorating quality of the Malaysian environment and also enable us to bequeath upon our future generation a cleaner, greener