Evidence from Direct Observations Using the Optical Visualization Technique
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Insecta: Coleoptera: Scarabaeidae : Cetoniinae : Tribe, Trichiini)1 Brandon Jones and Andrea Lucky2
EENY-704 Delta Flower Beetle Trigonopeltastes delta (Forster 1771) (Insecta: Coleoptera: Scarabaeidae : Cetoniinae : Tribe, Trichiini)1 Brandon Jones and Andrea Lucky2 Introduction The delta flower beetle, Trigonopeltastes delta (Forster), is a member of the scarab beetle family Scarabaeidae, in the subfamily Cetoniinae. This subfamily is commonly known as flower or fruit chafers because their diet consists mostly of decomposing fruits or pollen (Cave and Ratcliffe 2008). Trigonopeltastes delta belongs to the tribe Trichiini, which contains mostly flower-frequenting species. Although this species is commonly encountered where it occurs, many details of its life cycle and its potential economic importance remain poorly studied. Like many other cetoniines, the delta flower beetle has bright colors and distinctive patterns that distinguish it from other similar species (Figure 1). The delta flower beetle is one of two species in Florida, but while Trigonopeltastes delta is a familiar sight, Trigono- Figure 1. Adult Trigonopeltastes delta (Forster) (dorsal view). peltastes floridana is extremely rare (Woodruff 1960). While Credits: Mike Quinn, TexasEnto.net they are superficially similar, these species are distinguished by distinctive yellow markings on the pronotum. Trigono- Etymology and Synonymy peltastes delta bears a triangular mark whereas Trigono- The name Trigonopeltastes delta is Greek in origin and peltastes floridana has a U- or V-shaped mark. describes the pronotal markings of the species. Trigon translates to triangle, pelt translates to a shield, and delta originates from the letter Δ, or delta. The Greek symbol for delta is a triangle, which resembles the beetle’s pronotal marking, and accounts for its common name. 1. This document is EENY-704, one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. -

Approved Plant List 10/04/12
FLORIDA The best time to plant a tree is 20 years ago, the second best time to plant a tree is today. City of Sunrise Approved Plant List 10/04/12 Appendix A 10/4/12 APPROVED PLANT LIST FOR SINGLE FAMILY HOMES SG xx Slow Growing “xx” = minimum height in Small Mature tree height of less than 20 feet at time of planting feet OH Trees adjacent to overhead power lines Medium Mature tree height of between 21 – 40 feet U Trees within Utility Easements Large Mature tree height greater than 41 N Not acceptable for use as a replacement feet * Native Florida Species Varies Mature tree height depends on variety Mature size information based on Betrock’s Florida Landscape Plants Published 2001 GROUP “A” TREES Common Name Botanical Name Uses Mature Tree Size Avocado Persea Americana L Bahama Strongbark Bourreria orata * U, SG 6 S Bald Cypress Taxodium distichum * L Black Olive Shady Bucida buceras ‘Shady Lady’ L Lady Black Olive Bucida buceras L Brazil Beautyleaf Calophyllum brasiliense L Blolly Guapira discolor* M Bridalveil Tree Caesalpinia granadillo M Bulnesia Bulnesia arboria M Cinnecord Acacia choriophylla * U, SG 6 S Group ‘A’ Plant List for Single Family Homes Common Name Botanical Name Uses Mature Tree Size Citrus: Lemon, Citrus spp. OH S (except orange, Lime ect. Grapefruit) Citrus: Grapefruit Citrus paradisi M Trees Copperpod Peltophorum pterocarpum L Fiddlewood Citharexylum fruticosum * U, SG 8 S Floss Silk Tree Chorisia speciosa L Golden – Shower Cassia fistula L Green Buttonwood Conocarpus erectus * L Gumbo Limbo Bursera simaruba * L -
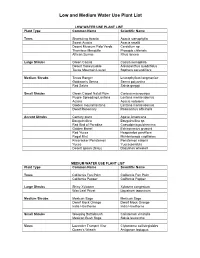
Low and Medium Water Use Plant List
Low and Medium Water Use Plant List LOW WATER USE PLANT LIST Plant Type Common Name Scientific Name Trees Shoestring Acacia Acacia stenophylla Sweet Acacia Acacia smallii Desert Museum Palo Verde Cercidium sp. Thornless Mesquite Prosopis chilensis African Sumac Rhus lancea Large Shrubs Green Cassia Cassia nemophila Desert Honeysuckle Anisacanthus quadrifidus Texas Mountain Laurel Sophora secundiflora Medium Shrubs Texas Ranger Leucophyllum langmaniae Goldman’s Senna Senna polyantha Red Salvia Salvia greggii Small Shrubs Green Carpet Natal Plum Carissa macrocarpa Purple Spreading Lantana Lantana montevidensis Acacia Acacia redolens Golden mound lantana Lantana montevidensis Dwarf Rosemary Rosmarinus officinalis Accent Shrubs Century plant Agave Americana Bougainvillea Bougainvillea sp. Red Bird of Paradise Caesalpinia pulcherrima Golden Barrel Echinocactus grusonii Red Yucca Hesperaloe parviflora Regal Mist Muhlenbergia capillaries Firecracker Penstemon Penstemon eatonii Yucca Yucca pendula Desert spoon (Grey) Dasylirion wheeleri MEDIUM WATER USE PLANT LIST Plant Type Common Name Scientific Name Trees California Fan Palm California Fan Palm California Pepper California Pepper Large Shrubs Shiny Xylosma Xylosma congestum Wax Leaf Privet Ligustrum japonicum Medium Shrubs Mexican Sage Mexican Sage Dwarf Mock Orange Dwarf Mock Orange India Hawthorne India Hawthorne Small Shrubs Weeping Bottlebrush Calistemon viminalis Mexican Bush Sage Salvia leucantha Vines Lavender Trumpet Vine Clytostoma callistegioides Queen’s Wreath Antigonon leptopus . -

Pruning Trees and Shrubs-2
Pruning Trees and Shrubs Ursula Schuch University of Arizona School of Plant Sciences 1 Publications on Pruning Pruning Deciduous Shade Trees http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1139.pdf Pruning Citrus http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1455.pdf Pruning Shrubs in the Low and Mid Elevation Deserts in Arizona http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1499.pdf 2 1 Pruning is the intentional removal of parts of a plant for a purpose. 3 Pruning Equipment Sterilizing tools Virus, vascular fungus, bacteria Avoid oozing cankers 70% Isopropyl alcohol Listerine, Lysol, Pine-Sol 4 2 Why Do We Prune Plants? To remove damaged/broken branches To remove rubbing, crossing, inwardly growing branches For visibility & safety considerations To train young plants Control plant size Rejuvenation of plants Increase flowering, fruiting and vigor 5 Dead branches Sign blocked Broken branch Rubbing/crossing branches 6 3 Co-dominant leader 7 Remove suckers, root stock suckers, and adventitious buds growing on the stem 8 4 Pruning Basics • Never remove more than 25‐30% of the canopy in any given year • If the plant requires frequent pruning, then it may not be the best suited plant for that situation • Usually best done in the early life of the plant • Shearing of shrubs is labor intensive, generally unnecessary, requires regular repeat shearing 9 Pruning Basics Heading versus Thinning Cut anywhere Leaves STUBS Cut back to next lateral branch NO STUBS 10 5 Where to cut? Opposite buds Above a bud when possible. Prevents unsightly stubs that die back. -

Taxonomic Overview of Ligustrum (Oleaceae) Naturalizaed in North America North of Mexico
Phytologia (December 2009) 91(3) 467 TAXONOMIC OVERVIEW OF LIGUSTRUM (OLEACEAE) NATURALIZAED IN NORTH AMERICA NORTH OF MEXICO Guy L. Nesom 2925 Hartwood Drive Fort Worth, TX 76109, USA www.guynesom.com ABSTRACT A key, morphological descriptions, and basic synonymy are provided for the eight species of Ligustrum known to be naturalized in North America north of Mexico: L. japonicum, L. lucidum, L. obtusifolium (including L. amurense), L. ovalifolium, L. quihoui, L. sinense, L. tschonoskii, and L. vulgare. Identifications have been inconsistent particularly between L. sinense and L. vulgare and between L. japonicum and L. lucidum. The occurrence of L. quihoui outside of cultivation in Arkansas, Mississippi, and Oklahoma is documented. Phytologia 91(3): 467-482 (December, 2009). KEY WORDS: Ligustrum, Oleaceae, North America, naturalized, taxonomy The lustrous, mostly evergreen leaves and masses of white, fragrant flowers make privets popular for landscaping and hedges. Many of the species, however, have become naturalized in the USA and Canada and already have proved to be destructive colonizers, especially in the Southeast. Among the naturalized species, European privet (Ligustrum vulgare) is native to Europe and northern Africa; all the rest are native to Asia, mainly China, Japan, and Korea. Many new species and varieties of Ligustrum have been described since overviews of Koehne (1904), Lingelsheim (1920), and Mansfield (1924). The genus in eastern Asia has recently been studied by Chang & Miao (1986), and Qin (2009) has provided a taxonomic overview of the whole genus that recognizes 37 species - divided into five sections based primarily on fruit and seed morphology. In Qin’s arrangement, among the North American species, sect. -

Non-Native Invasive Plants of the City of Alexandria, Virginia
March 1, 2019 Non-Native Invasive Plants of the City of Alexandria, Virginia Non-native invasive plants have increasingly become a major threat to natural areas, parks, forests, and wetlands by displacing native species and wildlife and significantly degrading habitats. Today, they are considered the greatest threat to natural areas and global biodiversity, second only to habitat loss resulting from development and urbanization (Vitousek et al. 1996, Pimentel et al. 2005). The Virginia Department of Conservation and Recreation has identified 90 non-native invasive plants that threaten natural areas and lands in Virginia (Heffernan et al. 2014) and Swearingen et al. (2010) include 80 plants from a list of nearly 280 non-native invasive plant species documented within the mid- Atlantic region. Largely overlapping with these and other regional lists are 116 species that were documented in the City of Alexandria, Virginia during vegetation surveys and natural resource assessments by the City of Alexandria Dept. of Recreation, Parks, and Cultural Activities (RPCA), Natural Lands Management Section. This list is not regulatory but serves as an educational reference informing those with concerns about non-native invasive plants in the City of Alexandria and vicinity, including taking action to prevent the further spread of these species by not planting them. Exotic species are those that are not native to a particular place or habitat as a result of human intervention. A non-native invasive plant is here defined as one that exhibits some degree of invasiveness, whether dominant and widespread in a particular habitat or landscape or much less common but long-lived and extremely persistent in places where it occurs. -

Vegetation Community Monitoring at Ocmulgee National Monument, 2011
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 ON THE COVER Duck potato (Sagittaria latifolia) at Ocmulgee National Monument. Photograph by: Sarah C. Heath, SECN Botanist. Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 Sarah Corbett Heath1 Michael W. Byrne2 1USDI National Park Service Southeast Coast Inventory and Monitoring Network Cumberland Island National Seashore 101 Wheeler Street Saint Marys, Georgia 31558 2USDI National Park Service Southeast Coast Inventory and Monitoring Network 135 Phoenix Road Athens, Georgia 30605 September 2014 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed. Consequently, the initial analyses of data in this report are provisional and subject to change. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Identification and Control of Invasive Privets (Ligustrum Spp.) in the Middle Southern United States
Invasive Plant Science and Management 2010 3:482–488 Notes and Commentary Identification and Control of Invasive Privets (Ligustrum spp.) in the Middle Southern United States Victor Maddox, John Byrd, Jr., and Brett Serviss* The identification of privet in the middle southern United States can be difficult. Because most introduced species of privet can be invasive, and recent mapping projects seek location and species population data, proper identification is important. Without proper identification of privet species, data on species distributions and other pertinent information regarding invasiveness could lead to improper conclusions. Currently, information on privet identification is scattered throughout a number of reference materials. The purpose of this publication is to assist with the proper identification of escaped privet species, and suggest management options. Nomenclature: Fosamine ammonium; glyphosate; hexazinone; imazapyr; metsulfuron; triclopyr; 2,4-D; 2,4-DP; Amur privet, Ligustrum obtusifolium Sieb. & Zucc. var. suave (Kitagawa) Kitagawa (Syn. L. amurense Carrie`re); border privet, Ligustrum obtusifolium Sieb. & Zucc. var. obtusifolium; California privet, Ligustrum ovalifolium Hassk.; Chinese privet, Ligustrum sinense Lour.; common privet, Ligustrum vulgare L.; glossy privet, Ligustrum lucidum Ait.; Japanese privet, Ligustrum japonicum Thunb.; waxyleaf privet, Ligustrum quihoui Carrie`re. Key words: Invasive species, management. Since the 1700s, at least nine species of privets have been Thunb.) is native to Korea and Japan. The most common introduced into the United States; it is probable that all species in the southern portion of the middle southern were introduced as ornamentals. They have been very region is Chinese privet, although Amu, border, California, successful as ornamentals and continue to be marketed for common or European, glossy, Japanese, and waxyleaf or such purposes. -

Ligustrum Japonicum Japanese Privet1 Edward F
Fact Sheet ST-352 November 1993 Ligustrum japonicum Japanese Privet1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Although often used as a shrub or hedge, Japanese Privet works well when allowed to grow into a small tree, its curved multiple trunks and dark green canopy creating an interesting architectural focus, 8 to 12 feet tall and often considerably wider, for the landscape (Fig. 1). Old specimens can grow to 25 feet across. The glossy evergreen leaves are abundantly produced on the upright, spreading branches. The small, white, malodorous flowers appear in terminal panicles during spring in the south and in the summer in northern climes. The blooms are followed by abundant blue- black berries which persist most of the year. The Figure 1. Mature Japanese Privet. berries are popular with birds and the dispersed seeds occasionally germinate where they fall but this is usually not a nuisance. lawns (3-4 feet wide); specimen; sidewalk cutout (tree pit); residential street tree; no proven urban tolerance GENERAL INFORMATION Availability: generally available in many areas within its hardiness range Scientific name: Ligustrum japonicum Pronunciation: lih-GUS-trum juh-PAWN-ih-kum DESCRIPTION Common name(s): Japanese Privet, Wax-Leaf Privet Family: Oleaceae Height: 8 to 12 feet USDA hardiness zones: 7B through 10A (Fig. 2) Spread: 15 to 25 feet Origin: not native to North America Crown uniformity: symmetrical canopy with a Uses: Bonsai; container or above-ground planter; regular (or smooth) outline, and individuals have more hedge; -

Ligustrum Spp
Species: Ligustrum spp. http://www.fs.fed.us/database/feis/plants/shrub/ligspp/all.html SPECIES: Ligustrum spp. Choose from the following categories of information. Introductory Distribution and occurrence Botanical and ecological characteristics Fire ecology Fire effects Management considerations References INTRODUCTORY SPECIES: Ligustrum spp. Japanese privet Chinese privet AUTHORSHIP AND CITATION FEIS ABBREVIATION SYNONYMS NRCS PLANT CODE J. S. Peterson, USDA, NRCS PLANTS Database Larry Allain, USDA, NRCS PLANTS Datab COMMON NAMES Amur privet European privet TAXONOMY LIFE FORM FEDERAL LEGAL STATUS OTHER STATUS Jon T. Lindstrom, Univ. of Arkansas ©Univ. Connecticut Plant Database AUTHORSHIP AND CITATION: Munger, Gregory T. 2003. Ligustrum spp. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: 1 of 26 9/24/2007 4:39 PM Species: Ligustrum spp. http://www.fs.fed.us/database/feis/plants/shrub/ligspp/all.html http://www.fs.fed.us/database/feis/ [2007, September 24]. FEIS ABBREVIATION: LIGSPP LIGAMU LIGJAP LIGSIN LIGVUL SYNONYMS: None NRCS PLANT CODE [62]: LIGUS2 LIAM LIJA LISI LIVU COMMON NAMES: Amur privet Japanese privet Chinese privet European privet common privet TAXONOMY: The currently accepted genus name for privet is Ligustrum L. (Oleaceae) [3,19,27,37,43,54,60,62,71,74,75]. This report summarizes information on 4 species of privet: Ligustrum amurense Carr. [27] Amur privet Ligustrum japonicum Thunb. [9,11,20,27,43,60,67,75] Japanese privet Ligustrum sinense Lour. [9,11,20,27,43,59,74,75] Chinese privet Ligustrum vulgare L. [3,19,25,27,37,54,60,69,71] European privet When discussing characteristics common to all 4 species, this report refers to them collectively as privet or privets. -

Japanese Privet.Pdf
Forest Management Sheet INVASIVE SPECIES http://texasforestservice.tamu.edu Japanese Privet PLANT: Japanese privet (Ligustrum japonicum) is an evergreen shrub with a spreading canopy, appearing as a single plant or a thicket. IDENTIFICATION: Evergreen, single plant or thicket- forming shrub with spreading canopy to 35 feet tall, thick opposite leaves, both leaves and stems hairless. Leaves are evergreen and leathery, opposite, ovate, 2-4 in. long and 1- 1.8 in. wide. Clusters of showy white flowers in spring yield dangling green to purple fruit in summer to winter. Similar to Chinese privet, only larger with larger leaves. It also resembles redtip. ECOLOGY: It occurs on same habitats as Chinese privet, but generally not as abundant depending upon location. Widely planted as an ornamental and escaped. It invades both lowland and upland habitats. Spreads by abundant animal-dispersed seeds and colonizes by rootsprouts. HERBICIDE CONTROL: Apply Garlon 4 as a 3% solution (12 ounces per 3-gal. mix) or Arsenal AC as a 1% solution (4 ounces per 3-gal. mix) in water with a surfactant to thoroughly wet all leaves in August through September. Apply a glyphosate herbicide as a 3% solution (12 ounces per 3-gal. mix) in water with a surfactant in March to June. For stems too tall for foliar sprays, apply a Garlon 4 as a 20% solution (2 quarts per 3-gal. mix) in commercially available basal oil, diesel fuel, or kerosene with a penetrant (check with herbicide distributor) to young bark as a basal spray. Cut large stems and treat stumps immediately after cutting with 10% solutions of Arsenal AC, Chopper, or Velpar L (1 quart per 3-gal. -

County of Riverside Friendly Plant List
ATTACHMENT A COUNTY OF RIVERSIDE CALIFORNIA FRIENDLY PLANT LIST PLANT LIST KEY WUCOLS III (Water Use Classification of Landscape Species) WUCOLS Region Sunset Zones 1 2,3,14,15,16,17 2 8,9 3 22,23,24 4 18,19,20,21 511 613 WUCOLS III Water Usage/ Average Plant Factor Key H-High (0.8) M-Medium (0.5) L-Low (.2) VL-Very Low (0.1) * Water use for this plant material was not listed in WUCOLS III, but assumed in comparison to plants of similar species ** Zones for this plant material were not listed in Sunset, but assumed in comparison to plants of similar species *** Zones based on USDA zones ‡ The California Friendly Plant List is provided to serve as a general guide for plant material. Riverside County has multiple Sunset Zones as well as microclimates within those zones which can affect plant viability and mature size. As such, plants and use categories listed herein are not exhaustive, nor do they constitute automatic approval; all proposed plant material is subject to review by the County. In some cases where a broad genus or species is called out within the list, there may be multiple species or cultivars that may (or may not) be appropriate. The specific water needs and sizes of cultivars should be verified by the designer. Site specific conditions should be taken into consideration in determining appropriate plant material. This includes, but is not limited to, verifying soil conditions affecting erosion, site specific and Fire Department requirements or restrictions affecting plans for fuel modifications zones, and site specific conditions near MSHCP areas.