Initial Burst of Root Development in Whole Fagus Crenata Seedlings: the Key to Survival?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
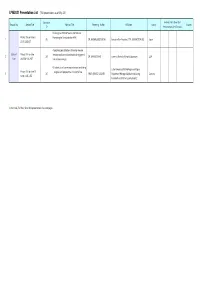
LPM2021 Presentation List (150 Presentations As of May 25 )
LPM2021 Presentation List (150 presentations as of May 25 ) Submission Invited / Full / Short Oral Session No. Session Title Abstract Title Presenting Author Affiliation County Student ID Presentations (On-Demand) Challenge to LPP-EUV Source and Material Plenary Talk on June 8 Processing for Semiconductor HVM 1 206 DR. HAKARU MIZOGUCHI Executive Vice President, CTO, GiGAPHOTON INC. Japan 21:15-22:00 JST A spectroscopic detection scheme to improve Session 1 Plenary Talk on June femtosecond laser induced molecular alignment 2 205 DR. XIANGLEI MAO Lawrence Berkeley National Laboratory USA Live AM 5:00-5:45, PDT and rotation analysis Ultrashort pulse laser micro and macro machining Leiter Kompetenzfeld Abtragen und Fügen, Plenary Talk on June 10 – progress and perspectives in industrial use 3 207 PROF. ARNOLD GILLNER Department Manager Ablation and Joining, Germany 14:00-14:45, CEST Fraunhofer-Institut für Lasertechnik ILT Invited talks, Full Oral / Short Oral presentations: See next pages. LPM2021 Presentation List (150 presentations as of May 25 ) Submission Invited / Full / Short Oral Session No. Session Title Abstract Title Presenting Author Affiliation County Student ID Presentations (On-Demand) First-principles calculations of initial stage of laser Fundamental aspects damage 4 Session 2 (Dynamics, modeling, 76 DR. ATSUSHI YAMADA University of Tsukuba Japan Invited simulation, etc.) Ultrashort laser heating of metals: insights from Fundamental aspects reflectivity and ablation threshold measurements 5 Session 2 (Dynamics, modeling, 19 DR. OLIVIER UTÉZA Aix-Maraseille University, CNRS, LP3 France Full simulation, etc.) Formation and Its Mechanism of High-speed Fundamental aspects Micro-grooving on Metal Surface by Angled CW 6 Session 2 (Dynamics, modeling, 101 MS. -

Gaiyoueng18.Pdf
YAMAGATAYAMAGATA UNIVERSITYUNIVERSITY INFORMATION BULLETIN OF THE NATIONAL UNIVERSITY CORPORATION YAMAGATA UNIVERSITY CONTENTS 1 WELCOME TO YAMAGATA UNIVERSITY 2 TOPICS 3 4 GENERAL EDUCATION FACULTY OF LITERATURE 5 6 AND SOCIAL SCIENCES 7 8 FACULTY OF EDUCATION, ART AND SCIENCE 9 10 FACULTY OF SCIENCE 11 12 FACULTY OF MEDICINE 13 14 FACULTY OF ENGINEERING 15 16 FACULTY OF AGRICULTURE 17 18 UNIVERSITY LIBRARY 19 UNIVERSITY HOSPITAL 20 GRADUATE SCHOOLS COMMON FACILITIES FOR EDUCATION 21 22 AND RESEARCH 23 24 INTERNATIONAL EXCHANGE PROGRAMS 25 26 CAMPUS LIFE 27 28 CAMPUS MAPS / TRANSPORTATION 29 30 YAMAGATA PREFECTURE WELCOME TO YAMAGATA UNIVERSITY 1 WELCOM E TO YAM AGATA UNIVERSITY MOVING TOWARDS A NEW YAMAGATA UNIVERSITY THAT STRENGTHENS ITS POSITION INTERNATIONALLY WHILE STILL INVOLVING THE LOCAL COMMUNITY Yamagata University consists of six faculties and was founded in 1949, during the post-war Japanese educational system reforms, through the amalgamation of five older colleges. The university has been incorporated and reborn as the National University Corporation Yamagata University in the April 2004 national university SENDO Fujiro legislative reform. We have since been as actively involved in academic activities as President ever and have succeeded in obtaining several Ministry of Education, Culture, Sports, Science and Technology (MEXT) grants, including one from the 21st century COE Program, and four from the Good Practice Program. In addition, we have been further strengthening our ties with the local community. Responding to strong demand from the northern Mogami region of the prefecture, where there are no higher education institutions, we have set up the whole region as a university campus and named it the Yamagata University Area Campus Mogami. -

Outline of IWATE UNIVERSITY for International Students a Wide Variety of Research Topics, Made Possible by the Extensive Campus
Outline of I ATE UNIVERSITY for International Students Contact Information Support available in Japanese, English, Chinese, and Korean International Office YouTube 3-18-34 Ueda, Morioka-shi, Iwate 020-8550 Japan TEL+81-19-621-6057 / +81-19-621-6076 FAX+81-19-621-6290 E-mail: [email protected] Website Instagram Support available only in Japanese Topic Division/Office in Charge TEL E-mail General Administration and Public Relations About the university in general Division, General Administration Department 019-621-6006 [email protected] Admissions Office, About the entrance exam Student Services Department 019-621-6064 Student Support Division, Facebook About student life Student Services Department 019-621-6060 [email protected] About careers for students Career Support Division, INDEX after graduation Student Services Department 019-621-6709 [email protected] Graduation certificates for graduates and Student Services Division, 1. About Iwate University ………………………………… p.2 students who have completed their studies Student Services Department 019-621-6055 [email protected] 2. Undergraduate and Graduate Programs ………… p.4 3. Research Topics ………………………………………… p.14 Twitter 4. Types of International Students …………………… p.16 5. Support for International Students ……………… p.18 Website Iwate University Japanese English https://www.iwate-u.ac.jp/english/index.html 6. A Day in the Life of an International Student… p.20 Global Education Center Japanese English Chinese Korean https://www.iwate-u.ac.jp/iuic/ 7. Interviews with International Students ………… p.22 Researchers Database Japanese English http://univdb.iwate-u.ac.jp/openmain.jsp 8. Campus Calendar………………………………………… p.23 Questions related to the entrance exam Japanese https://www.iwate-u.ac.jp/admission/index.html WeChat (Chinese International Students Association) 9. -

Graduate School of Science and Engineering YAMAGATA
ProfileProfile ofof thethe TohokuTohoku MITRAIMITRAI CourseCourse (for(for internationalinternational students)students) GraduateGraduate SchoolSchool ofof ScienceScience andand EEngineeringngineering (Engineer(Engineer FField)ield), YamagataYamagata UniversityUniversity CoursesCourses ooff SSpecializationpecialization Master’s program(Engineering) Doctoral program(Engineering) In addition to the student’s research in their field of specialty, the Management of Technology for Manufacturing (MOT) Major, Tohoku MITRAI Course systematically provides seminars, special education, management of technology education, and Japanese society education to develop human resources that can be actively involved in global business activity and business expansion. For students who wish to work in a Japanese company after graduation, the provides excellent career guidance based on previous experience and Organic Device Engineering results and carefully supports international students in all aspects of their career planning and placement. Students who complete this This course provides with consistent research systems from synthesis course will receive a Master’s in Engineering. of organic electronic materials to fabrication and valuation of organic devices. The course also aims at rearing up the researchers to lead the global dedication besides fundamental researches. Organic Materials Engineering Yamagata University Graduate School, Tohoku MITRAI Course This major provides students with following divergent research Seminars Special Education SpecializedSpecialized -

1065 RADIOCARBON LABORATORIES This Is
RADIOCARBON LABORATORIES This is Radiocarbon’s annual list of active radiocarbon laboratories and personnel known to us. Conventional beta-counting facilities are listed in Part I, and accelerator mass spectrometry (AMS) facilities are listed in Part II. Laboratory code designations, used to identify published dates, are given to the left of the listing. (See p 1086 for a complete list of past and present lab codes.) Please notify us of any changes in staff, addresses, or other contact information. I. CONVENTIONAL 14C COUNTING FACILITIES ARGENTINA AC Héctor Osvaldo Panarello Pabellón INGEIS Ciudad Universitaria 1428 Buenos Aires, Argentina Tel: +54 11 4783 3021/23; Fax: +54 11 4783 3024 Email: [email protected] http://www.ingeis.uba.ar/ LP Jorge E. Carbonari and Roberto A. Huarte Laboratorio de Tritio y Radiocarbono, LATYR Centro de Investigaciones Geológicas, CIG Facultad de Ciencias Naturales y Museo, UNLP Paseo del Bosque s/n. (1900) La Plata, Argentina Tel: + 54 221 4270648 Email: [email protected] AUSTRIA IAEA Manfred Gröning International Atomic Energy Agency (IAEA) Isotope Hydrology Laboratory Wagramerstrasse 5 P.O. Box 100 A-1400 Vienna, Austria Tel: +43 1 2600 21740/21766; Fax: +43 1 2600 7 Email: [email protected] http://www.iaea.org/programmes/rial/pci/isotopehydrology/ BELARUS IGSB N. D. Michailov Institute of Geochemistry and Geophysics of the National Academy of Sciences of Belarus Kuprevich str. 7 Minsk 220141, Belarus Tel: +375 (17) 263 81 13; Fax: +375 (17) 263 63 98 Email: [email protected] BRAZIL CENA Luiz Carlos Ruiz Pessenda Radiocarbon Laboratory Centro de Energia Nuclear na Agricultura Universidade de São Paulo Avenida Centenario 303 Caixa Postal 96 – CEP 13400-970 Piracicaba, São Paulo, Brazil Tel: +55 193 429 4656; Fax: +55 193 429 4610 Email: [email protected] http://www.cena.usp.br/divisoes-cientificas/desenvolvimento-metodos-tecnicas-analiticas-nucleares/laboratorio/ carbono-14 1065 1066 Laboratories FZ M. -

College Codes (Outside the United States)
COLLEGE CODES (OUTSIDE THE UNITED STATES) ACT CODE COLLEGE NAME COUNTRY 7143 ARGENTINA UNIV OF MANAGEMENT ARGENTINA 7139 NATIONAL UNIVERSITY OF ENTRE RIOS ARGENTINA 6694 NATIONAL UNIVERSITY OF TUCUMAN ARGENTINA 7205 TECHNICAL INST OF BUENOS AIRES ARGENTINA 6673 UNIVERSIDAD DE BELGRANO ARGENTINA 6000 BALLARAT COLLEGE OF ADVANCED EDUCATION AUSTRALIA 7271 BOND UNIVERSITY AUSTRALIA 7122 CENTRAL QUEENSLAND UNIVERSITY AUSTRALIA 7334 CHARLES STURT UNIVERSITY AUSTRALIA 6610 CURTIN UNIVERSITY EXCHANGE PROG AUSTRALIA 6600 CURTIN UNIVERSITY OF TECHNOLOGY AUSTRALIA 7038 DEAKIN UNIVERSITY AUSTRALIA 6863 EDITH COWAN UNIVERSITY AUSTRALIA 7090 GRIFFITH UNIVERSITY AUSTRALIA 6901 LA TROBE UNIVERSITY AUSTRALIA 6001 MACQUARIE UNIVERSITY AUSTRALIA 6497 MELBOURNE COLLEGE OF ADV EDUCATION AUSTRALIA 6832 MONASH UNIVERSITY AUSTRALIA 7281 PERTH INST OF BUSINESS & TECH AUSTRALIA 6002 QUEENSLAND INSTITUTE OF TECH AUSTRALIA 6341 ROYAL MELBOURNE INST TECH EXCHANGE PROG AUSTRALIA 6537 ROYAL MELBOURNE INSTITUTE OF TECHNOLOGY AUSTRALIA 6671 SWINBURNE INSTITUTE OF TECH AUSTRALIA 7296 THE UNIVERSITY OF MELBOURNE AUSTRALIA 7317 UNIV OF MELBOURNE EXCHANGE PROGRAM AUSTRALIA 7287 UNIV OF NEW SO WALES EXCHG PROG AUSTRALIA 6737 UNIV OF QUEENSLAND EXCHANGE PROGRAM AUSTRALIA 6756 UNIV OF SYDNEY EXCHANGE PROGRAM AUSTRALIA 7289 UNIV OF WESTERN AUSTRALIA EXCHG PRO AUSTRALIA 7332 UNIVERSITY OF ADELAIDE AUSTRALIA 7142 UNIVERSITY OF CANBERRA AUSTRALIA 7027 UNIVERSITY OF NEW SOUTH WALES AUSTRALIA 7276 UNIVERSITY OF NEWCASTLE AUSTRALIA 6331 UNIVERSITY OF QUEENSLAND AUSTRALIA 7265 UNIVERSITY -
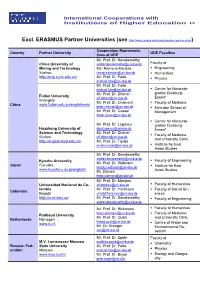
List of International University Cooperations (PDF)
International Cooperations with Institutions of Higher Education 1/8 Excl. ERASMUS Partner Universities (see http://www.uni-due.de/international/en_partner.shtml) Cooperation Representa- Country Partner University UDE Faculties tives at UDE Mr. Prof. Dr. Kecskeméthy China University of [email protected] Faculty of Mining and Technology Ms. Ramona Karatas . Engineering Xuzhou [email protected] . Humanities http://eng.cumt.edu.cn/ Mr. Prof. Dr. Farle . Physics [email protected] Mr. Prof. Dr. Farle [email protected] . Center for Nanointe- Mr. Prof. Dr. Dittmer gration Duisburg- Fudan University [email protected] Essen* Shanghai Mr. Prof. Dr. Chamoni . Faculty of Medicine China www.fudan.edu.cn/englishnew/ [email protected] . Mercator School of Mr. Prof. Dr. Cassel Management [email protected] . Center for Nanointe- Mr. Prof. Dr. Lupascu gration Duisburg- Huazhong University of [email protected] Essen* Science and Technology Mr. Prof. Dr. Dittmer . Faculty of Medicine Wuhan [email protected] and University Clinic http://english.hust.edu.cn/ Mr. Prof. Dr. Taube [email protected] . Institute for East Asian Studies Mr. Prof. Dr. Kecskeméthy [email protected] Kyushu University . Faculty of Engineering Mr. Prof. Dr. Widmann Japan Fukuoka [email protected] . Institute for East www.kyushu-u.ac.jp/english/ Mr. Demes Asian Studies [email protected] Mr. Prof. Dr. Monjour Universidad Nacional de Co- [email protected] . Faculty of Humanities lombia Mr. Prof. Dr. Hartmann . Faculty of Social Sci- Colombia Bogotá [email protected] ences http://unal.edu.co/ Mr. -

CURRICULUM VITAE ANDR´AS NÉMETHI Educational History
CURRICULUM VITAE ANDRAS´ NEMETHI´ Educational History Degrees Institution Dates Awarded BS University of Bucharest, Romania June 1983 MS University of Bucharest, Romania June 1984 Doctor in Math. Institute of Math. of Romanian Academy July 1990 Bucharest, Romania PhD The Ohio State University August 1991 Columbus, Ohio, USA ScD Hungarian Academy of Sciences 2001 Habilitation E¨otv¨osUniversity, Budapest, Hungary 2008 Professional Experience Institution Dates Nat. Inst. for Sc. and Tech. Research, Bucharest (Researcher) 1985 − 1990 OSU, Columbus, Ohio (Instructor) 1991 − 1995 OSU, Columbus, Ohio (Assistant Professor) 1995 − 1998 OSU, Columbus, Ohio (Associate Professor) 1998 − 2002 OSU, Columbus, Ohio (Professor) 2002 − 2007 R´enyi Institute of Math., Budapest, Hungary 1999 − 2001 R´enyi Institute of Math., Budapest, Hungary 2004− E¨otv¨osUniversity, Budapest, Hungary (Professor) 2008− External Scientific Member Basque Center for Applied Mathematics, Bilbao, Spain Reserach Visiting Positions Math. Inst. of the Hungarian Academy of Sciences March 1990{May, 1990 Universities of Utrecht and Nijmegen, Netherlands Sept. 1990{Dec. 1990 University of Toronto, Canada July 01{31, 1991 MSRI, Berkeley May 01{31, 1993 University of Nice, France July 01{31, 1993 University of Nijmegen, The Netherlands Sept. 1993{June, 1994 Ecole´ Polytechnique, Palaiseau, France Oct. 01{Dec. 31, 1996 University of Nice, France June 15{July 15, 1997 University of Nantes, France June 01{30, 1998 University of Bordeaux, France Nov 01{30, 1999 R´enyi Institute of Math., Budapest, Hungary July 1999- June 2000 University of Hannover, Germany Oct. 01{30, 2005 University of Nice, France May 01{30, 2007 University M´editerran´ee,Marseille, France Sept. 01{30, 2007 University of Yamagata, Yamagata, Japan Sept. -

東北 日本 the Program Consists Of: Participants: • Developing a System of International Seminars 1
Agriculture, Forestry and Clean Winter school in Energies in Yamagata and Miyagi Prefecture Japan 2019 Objectives 東北 日本 The program consists of: Participants: • Developing a system of international seminars 1. Lectures given by specialists from universi- on global issues among the partner ties, research institutes on multiple elds universities. ranging from forestry, agriculture, eective Yamagata University, Japan use of resources and application of technol- • Understanding the different point of views ogy in cold environments. involved in attaining sustainable development. Leibniz University Hannover, Balance between economic growth and 2. Visit a Clean Energy Power Plantation in Germany environment. Yamagata, a Restoration Park in Miyagi and • Understanding the link between field research coastal forests in both prefectures. Chiang Mai University, and its application. Thailand 3. Group discussion among students about the • Building a cross-cultural bridge that will topics presented in the program. contribute to develop a youth network among students. Vietnam National University 4. Presentations by students of the participat- of Agriculture, Vietnam • Promote students exchange among the ing universities to outline their focus of participating Universities. research. • Motivate students about the importance of 5. Cultural events and visit to historical sites understanding local as well as global issues. representative of northeastern Japan. Yamagata University Faculty of Agriculture Prof. Dr. M. Larry Lopez C. phone: (+81)-235-28-2961 email: [email protected] homepage: https://www.tr.yamagata-u.ac.jp/~larry/prole.html Schedule 04. MARCH | Mon February 25-08 08:10 Move to JRE (Japan Renewable Energy) 12:30 Lunch 25. FEBRUARY | Mon | Mon 13:30 Preparation of group presentations 09:20 Departure from Hotel 16:00 Group presentations 11:00 Explanation of Zenpoji 28. -

Program of Yamagata IOS.Pdf へのリンク
International Online Symposium on Soil C and N Dynamics by Land Use, Management and Climate Changes Main Topics: Carbon sequestration? Greenhouse gases emission? Plants production? Soil fertility? Effect of Climate Changes? Soil mineral and organic components? Date : September 28 ‐ 29, 2020 Venue: Zoom Cloud Meeting for most participants, and Yamagata University for participants who can attend in Tsuruoka, Japan Organizer & Contact: Prof. Weiguo CHENG (Yamagata University, Japan) Phone: +81‐235‐28‐2824 E‐mail: [email protected]‐u.ac.jp Sponsor: Yamagata University UGAS, Iwate University Program (1) OM: Opening message; KN: Keynote; OC: Oral Communication; P: Poster Presentation Monday, 2020/9/28 Time in Time in Presenter, Session / Presentation Presenter Japan Affiliation country 8:00 Start registration online Opening Ceremony OM 1 Opening welcome message from Dean of United Prof. UEMURA Japan Graduate School of Agricultural Sciences, Iwate Matsuo, Iwate 9:00-9:10 University University 9:00-9:10 Prof. MURAYAMA OM 2 Opening welcome message from Dean of Faculty Japan Hideki, Yamagata of Agriculture,Yamagata University 9:10-9:20 University 9:10-9:20 OM 3 Opening welcome message and introduction of the Prof. CHENG Weiguo, Japan 9:20-9:30 IOS from Organizer Yamagata University 9:20-9:30 Session 1: Natural and Artificial Wetlands KN 1 Effects of mire disturbance by Sika deer on Prof. INUBUSHI Japan physico-chemical properties of peat soils and Kazuyuki, Chiba 9:30-10:00 greenhouse gas flux in Ozegahara Mire, Japan University 9:30-10:00 USA KN 2 Soil and vegetation characteristics along Prof. Jay P. -

Program in Pdf Format Is Available Here
1 Messages from Chairs of IPOMY Our Graduate School of Organic Materials Science of Yamagata University (YU), Japan, is privileged and honored to hold The First International Conference of Polymeric and Organic Materials in Yamagata University (IPOMY) at Denkoku-no-Mori in Yonezawa, Japan, from January 24 to 26, 2019. This international conference is not only giving an opportunity for YU’s members and all invited worldwide researchers to present and discuss their research works and update their expertise, but also to initially stimulate the development of research works on the polymer, organic materials, and its related devices. Polymeric materials and functional organic materials are one of the most significant material groups driving the current and supporting the future society. Nowadays, the high performance and new functions of these materials are actively developing. In the future, it is also expected to create organic material systems with new value-added that maximizes the use of organic materials, considering integration with other fields and usage in the boundary area. Continued far-reaching and wide-ranging collaboration and exchange of ideas among the people in the area of polymers and organic materials, especially between our YU active members and world's leading researchers are essential for the creation of innovation in our field. We hope that everyone participating will build a new research network, start new research topics, and publish the co- authored papers. On behalf of the conference Organizing Committee, we welcome all delegates and wishing you all the best for an academically and professionally fruitful meeting. And we hope all delegates from abroad enjoy their stay in Yonezawa, Japan. -

Program Committee Ayumi Hirano-Iwata, Tohoku Univ
11th International Workshop on Nanostructures & Nanoelectronics Laboratory for Nanoelectronics and Spintronics Research Institute of Electrical Communication Tohoku University Organized by Research Institute of Electrical Communication Tohoku University Co-Organized by Nano-Spin Engineering Seminar Cooperative Research Projects Information Biotronics Seminar Cooperative Society CREST “Construction of ion and electron nano-channels in super-resistive lipid bilayers”, JST Division of Soft-Nanotechnology, The Japan Society of Vacuum and Surface Science March 5-6, 2020 Sendai, Japan 11th International Workshop on Nanostructures & Nanoelectronics March 5-6, 2020 Site: Laboratory for Nanoelectronics and Spintronics, Research Institute of Electrical Communication, Tohoku University, Sendai, Japan Organizer: Symposium Chairs Ayumi Hirano-Iwata, Tohoku Univ. Ryugo Tero, Toyohashi Univ. of Tech. Program Committee Ayumi Hirano-Iwata, Tohoku Univ. Organizing Committee Ayumi Hirano-Iwata, Tohoku Univ. Ryugo Tero, Toyohashi Univ. of Tech. Teng Ma, Tohoku Univ. Daisuke Tadaki, Tohoku Univ. Program March 5 (Thursday) Room: 4F, Conference Room, Laboratory for Nanoelectronics and Spintronics 14:00 ~ 14:05 Opening Remarks Ayumi Hirano-Iwata (Advanced Institute for Materials Research / Research Institute of Electrical Communication, Tohoku University, Sendai, Japan) (Chair: Ryugo Tero) 14:05 ~ 14:50 Sugar chain modified graphene FET for virus detection Kazuhiko Matsumoto (Institute of Scientific & Industrial Research, Osaka University, Japan) 14:50 ~ 15:35