C9an00927b1.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Chlorpyrifos, Part 1: Toxicology
JOURNAL OF PESTICIDE REFORM/ WINTER 1994 • VOL.14, NO. 4 ■ INSECTICIDE FACTSHEET CHLORPYRIFOS, PART 1: TOXICOLOGY The broad spectrum organophosphate insecticide chlorpyrifos is the most widely used insecticide in the U.S. Total use is estimated at almost 30 million pounds per year. Like all organophosphate insecticides, chlorpyrifos affects the nervous system by inhibiting an enzyme that is important in the transmission of nerve impulses. Symptoms of acute poisoning include headache, nausea, muscle twitching, and convulsions. Chlorpyrifos poisonings are reported to state and federal agencies more often than poisonings of almost every other insecticide. In both laboratory animals and humans, chlorpyrifos can also cause delayed effects on the nervous system. Some effects have been measured years after exposure. Human birth defects have been associated with exposure to chlorpyrifos products. In pregnant laboratory animals, chlorpyrifos exposure caused fetal death. Pups that did survive were smaller pups and did not survive as well as pups from unexposed mothers. Chlorpyrifos also affects the male reproductive system; exposure to a chlorpyrifos product has caused death of cells in male rat testes and a decrease in sperm production in cattle. Chlorpyrifos has caused genetic damage in human blood and lymph cells, mice spleen cells, and hamster bone marrow cells. Immune system abnormalities have been reported from patients exposed to chlorpyrifos. Many individuals report developing sensitivities to a broad array of substances following chlorpyrifos exposure. The second part of this factsheet will discuss human exposure to chlorpyrifos and the ecological effects of chlorpyrifos. BY CAROLINE COX mary agricultural uses are for oranges, al- plications are made annually. -

The List of Extremely Hazardous Substances)
APPENDIX A (THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES) THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 75-86-5 ACETONE CYANOHYDRIN 500 10 1752-30-3 ACETONE THIOSEMICARBAZIDE 500/500 1,000 107-02-8 ACROLEIN 500 1 79-06-1 ACRYLAMIDE 500/500 5,000 107-13-1 ACRYLONITRILE 500 100 814-68-6 ACRYLYL CHLORIDE 100 100 111-69-3 ADIPONITRILE 500 1,000 116-06-3 ALDICARB 100/500 1 309-00-2 ALDRIN 500/500 1 107-18-6 ALLYL ALCOHOL 500 100 107-11-9 ALLYLAMINE 500 500 20859-73-8 ALUMINUM PHOSPHIDE 500 100 54-62-6 AMINOPTERIN 500/500 500 78-53-5 AMITON 500 500 3734-97-2 AMITON OXALATE 100/500 100 7664-41-7 AMMONIA 500 100 300-62-9 AMPHETAMINE 500 1,000 62-53-3 ANILINE 500 5,000 88-05-1 ANILINE,2,4,6-TRIMETHYL- 500 500 7783-70-2 ANTIMONY PENTAFLUORIDE 500 500 1397-94-0 ANTIMYCIN A 500/500 1,000 86-88-4 ANTU 500/500 100 1303-28-2 ARSENIC PENTOXIDE 100/500 1 THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 1327-53-3 ARSENOUS OXIDE 100/500 1 7784-34-1 ARSENOUS TRICHLORIDE 500 1 7784-42-1 ARSINE 100 100 2642-71-9 AZINPHOS-ETHYL 100/500 100 86-50-0 AZINPHOS-METHYL 10/500 1 98-87-3 BENZAL CHLORIDE 500 5,000 98-16-8 BENZENAMINE, 3-(TRIFLUOROMETHYL)- 500 500 100-14-1 BENZENE, 1-(CHLOROMETHYL)-4-NITRO- 500/500 500 98-05-5 BENZENEARSONIC ACID 10/500 10 3615-21-2 BENZIMIDAZOLE, 4,5-DICHLORO-2-(TRI- 500/500 500 FLUOROMETHYL)- 98-07-7 BENZOTRICHLORIDE 100 10 100-44-7 BENZYL CHLORIDE 500 100 140-29-4 BENZYL CYANIDE 500 500 15271-41-7 BICYCLO[2.2.1]HEPTANE-2-CARBONITRILE,5- -

A Pesticide Decision-Making Guide to Protect Pollinators in Landscape
A Pesticide Decision-Making Guide to Protect Pollinators in Landscape, Ornamental and Turf Management 2019 Edition By Maria van Dyke, Emma Mullen, Dan Wixted, and Scott McArt Pollinator Network at Cornell, 2018 Cornell University, Department Of Entomology Download this guide for free from: https://pollinator.cals.cornell.edu/resources/grower-resources/ Contents Choosing lower-risk pesticides for pollinators in landscape, ornamental & turf management ____ 1 How to use this guide 3 Understanding the terms in this guide 4 EPA Pesticide toxicity standards 4 Synergistic Interactions 4 Systemic Pesticides 4 Adjuvants and/or inert ingredients 5 Tying it all together: adopting an Integrated Pest and Pollinator Management (IPPM) approach 5 IPPM: Putting the “pollinator” in IPM: 6 Table 1: Product formulations and their active ingredients 7 Table 2: Pesticide synergies and acute, chronic, and sublethal toxicities for honey bees and other pollinators 10 Literature cited 25 Appendix A: Pollination contract ______________________________________________________ 29 Acknowledgments This research and development of this guide was supported by the New York State Environmental Protection Fund and New York Farm Viability Institute grant FOC 17-001. The expert advice and consultation provided by Dan Wixted of the Cornell Pesticide Management Education Program was supported by the Crop Protection and Pest Management Extension Implementation Program [grant no. 2017-70006-27142/project accession no. 1014000] from the USDA National Institute of Food and Agriculture. 1 Choosing lower-risk pesticides for pollinators in landscape, ornamental & turf management Managing pests on ornamentals, in landscapes, and in nurseries while protecting pollinators can be a balancing act. Pollinators (mostly bees) are busy pollinating blossoms in nurseries and landscapes at the same time growers and landscapers need to be managing specific pests and diseases. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

UNITED NATIONS Stockholm Convention on Persistent Organic
UNITED NATIONS SC UNEP/POPS/POPRC.8/INF/12 Distr.: General 14 August 2012 English only Stockholm Convention on Persistent Organic Pollutants Persistent Organic Pollutants Review Committee Eighth meeting Geneva, 15–19 October 2012 Item 5 (e) and (f) of the provisional agenda* Technical work: assessment of alternatives to endosulfan; assessment of alternatives to DDT Report on the assessment of chemical alternatives to endosulfan and DDT Note by the Secretariat As referred to in documents UNEP/POPS/POPRC.8/8 and UNEP/POPS/POPRC.8/9, the report on the assessment of chemical alternatives to endosulfan and DDT is set out in the annex to the present note; it has not been formally edited. * UNEP/POPS/POPRC.8/1. K1282318 040912 UNEP/POPS/POPRC.8/INF/12 Annex Report on the assessment of chemical alternatives to endosulfan and DDT Draft prepared by the ad hoc working group on assessment of alternatives to endosulfan and DDT under the POPs Review Committee of the Stockholm Convention July 2012 2 UNEP/POPS/POPRC.8/INF/12 Table of Content 1. Disclaimer 2. Background and proposed results 3. Prioritization of Chemical Alternatives for Endosulfan with respect to the Persistent Organic Pollutant (POP) Characteristics (Annex D) 3.1. Introduction 3.2. Endpoint and data selection for prioritisation 3.3. Experimental information 3.4. QSAR information 3.5. Description of the data sources 3.6. Uncertainties 3.7. Data analysis 3.8. Results 3.9. Comments on selected alternative substances 4. Methodology for the assessment of persistent organic pollutant characteristics and identification of other hazard indicators for the assessment of chemical alternatives to Endosulfan and DDT 4.1. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Pesticide Reference Values Comparison Study
Is Protecting Aquatic Life from Pesticides Sufficient to Ensure Human Health Protection in Sources of Drinking Water? Kelly D. Moran, Ph.D., TDC Environmental, LLC Bonny Starr, P.E., Starr Consulting October 1, 2018 Abstract California water and pesticides regulators have long operated under the informal assumption that programs to protect aquatic life from currently used pesticides will also ensure the safety of surface water drinKing water sources. This paper examines the scientific validity of this assumption for the agricultural pesticides in California’s Central Valley by comparing water quality regulatory values and benchmarks (“reference values”) for human health with those for aquatic life. Because numeric water quality criteria and other numeric regulatory values established for water quality protection exist for only a handful of currently used pesticides, the comparison relies heavily on US EPA pesticides human health and aquatic life benchmarks. For acute endpoints, both human health and aquatic life reference values typically use a one-day exposure time frame, but chronic endpoint exposure periods differ, with aquatic life exposure periods (4 to 60 days) usually shorter than human health exposure periods (annual). The evaluation looKed in detail at 301 agricultural pesticides with human health reference values. Of these 301 pesticides, only 46% had aquatic life reference values that were equal to or lower than the human health reference value. For 54% of these pesticides, either no aquatic life reference value existed or the aquatic life reference value was higher than the human health reference value. In these cases, aquatic life protection actions would not suffice to protect human health. -
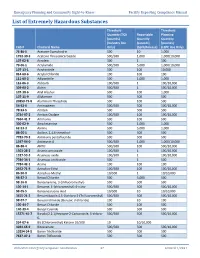
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Pesticide Resistance in Bed Bugs Everywhere!!!!!
2/24/2018 Pesticide Resistance in Bed bugs were virtually eradicated from the U.S. in Bed Bugs the post WWII era due to DDT and other powerful Shujuan (Lucy) Li insecticides. University of Arizona Alvaro Romero New Mexico State University 2 By the 1960s, bed bugs had developed resistance Public housing Apartments to DDT, methoxychlor and analogues, BHC, Schools dieldrin and analogues , and pyrethrins ( Busvine 1958, Hospitals Nursing homes Cwilich & Mer 1957, Mallis and Miller 1964 ) . Homes Transportation Child care Medical facilities Hotels & motels Health care facilities Airports Movie theaters Department stores Products, vendors, or commercial services mentioned or pictured in this seminar are for Everywhere!!!!! illustrative purposes only and are not meant to be endorsements. 3 4 University of Arizona; Arizona Pest Management Center 1 2/24/2018 Possible reasons for treatment failure? Missed some Clutter Reintroduction Have you seen these after treatments? 5 6 Dose - response assays for field - collected strains Bed bugs survived direct insecticide sprays 99 deltamethrin 90 Ft. Dix F1 50 ) e l a c 10 s t CIN1 i b o 1.0 r p ( y t i l a t r 99 - cyhalothrin o m e 90 g a t n Resistance ratio (RR) at least 6,000 !!! e c Ft. Dix r 50 e P 10 CIN1 Suspend® ( Deltamethrin ) 1.0 10 -7 10 -6 10 -5 10 -4 10 -3 10 -2 10 -1 10 0 10 1 10 2 10 3 10 4 Treatment (mg active ingredient/cm 2 ) Products, vendors, or commercial services mentioned or pictured in this seminar are for illustrative purposes only and are not meant Romero et al. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved.