Lassa Virus Circulation in Small Mammal Populations in Bo District, Sierra Leone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Linking Behavior, Co-Infection Patterns, and Viral Infection Risk with the Whole Gastrointestinal Helminth Community Structure in Mastomys Natalensis
ORIGINAL RESEARCH published: 17 August 2021 doi: 10.3389/fvets.2021.669058 Linking Behavior, Co-infection Patterns, and Viral Infection Risk With the Whole Gastrointestinal Helminth Community Structure in Mastomys natalensis Bram Vanden Broecke 1*, Lisse Bernaerts 1, Alexis Ribas 2, Vincent Sluydts 1, Ladslaus Mnyone 3, Erik Matthysen 1 and Herwig Leirs 1 1 Evolutionary Ecology Group, Department of Biology, University of Antwerp, Antwerp, Belgium, 2 Parasitology Section, Department of Biology, Healthcare and Environment, Faculty of Pharmacy and Food Science, IRBio (Research Institute of Biodiversity), University of Barcelona, Barcelona, Spain, 3 Pest Management Center, Sokoine University of Agriculture, Morogoro, Tanzania Edited by: Yadong Zheng, Infection probability, load, and community structure of helminths varies strongly between Lanzhou Institute of Veterinary and within animal populations. This can be ascribed to environmental stochasticity Research (CAAS), China or due to individual characteristics of the host such as their age or sex. Other, but Reviewed by: Mario Garrido, understudied, factors are the hosts’ behavior and co-infection patterns. In this study, we Ben-Gurion University of the used the multimammate mouse (Mastomys natalensis) as a model system to investigate Negev, Israel Si-Yang Huang, how the hosts’ sex, age, exploration behavior, and viral infection history affects their Yangzhou University, China infection risk, parasitic load, and community structure of gastrointestinal helminths. We Hannah Rose Vineer, hypothesized that the hosts’ exploration behavior would play a key role in the risk for University of Liverpool, United Kingdom infection by different gastrointestinal helminths, whereby highly explorative individuals *Correspondence: would have a higher infection risk leading to a wider diversity of helminths and a larger Bram Vanden Broecke load compared to less explorative individuals. -

Eastern Province
SIERRA LEONE EASTERN PROVINCE afi B or B a fi n Guinea Guinea KOINADUGU KAMBIA BOMBALI ! PORT LOKO KONO Fandaa TONKOLILI 5 ! Henekuma WESTERN AREA ! Dunamor ! ! Powma KAILAHUN Fintibaya ! Siakoro MOYAMBA BO ! Konkonia ! Kondewakor Kongowakor !! KENEMA ! ! Saikuya ! M!okeni K! ongoadu Bongema II ! Poteya ! ! ! T o l i ! Komandor T o l i Kombodu ! ! ! ! ! ! Kondeya Fabandu Foakor ! !! BONTHE Thomasidu Yayima Fanema ! Totor ! !! Bendu Leimaradu ! ! ! Foindu ! ! Gbolia PUJEHUN !!Feikaya Sakamadu ! ! Wasaya! Liberia Bayawaindu ! Bawadu Jongadu Sowadu ! Atlantic Ocean Norway Bettydu ! ! Kawamah Sandia! ! ! ! ! ! Kamindo ! !! ! ! Makongodu ! Sam! adu Bondondor ! ! Teiya ! ! ! ! Wonia ! Tombodu n Primary School ! ! e wordu D Wordu C Heremakonoh !! ! !! ! Yendio-Bengu ! d !! Kwafoni n ! !Dandu Bumanja !Kemodu ! ! e ! Yaryah B Yuyah Mor!ikpandidu ! ! S ! ! ! ! ! Kondeya II ! r ! ! ! ! !! Kindia o ! ! Deiyor! II Pengidu a Makadu Fosayma Bumbeh ! ! an Kabaidu ! Chimandu ! ! ! Yaryah A ! Kondeya Kongofinkor Kondeya Primary School ! Sambaia p ! ! ! F!aindu m Budu I !! Fodaydu ! ! Bongema I a !Yondadu !Kocheo ! Kwakoima ! Gbandu P Foimangadu Somoya ! ! Budu II ! ! ! Koyah ! Health Centre Kamba ! ! Kunundu Wasaya ! ! ! ! Teidu ! Seidu Kondeya 1 ! ! Kayima A ! Sandema! ! ! suma II suma I Primary School ! ! ! ! Kayima B Koidundae !!! R.C. Primary School ! ! ! !! Wokoro UMC Primary School !! Sangbandor Tankoro ! ! Mafidu ! Dugbema ! Piyamanday ! Suma I Bendu ! Kayima D ! ! Kwikuma Gbeyeah B ! Farma Bongema ! Koekuma ! Gboadah ! ! ! ! Masaia ! Gbaiima Kombasandidu -
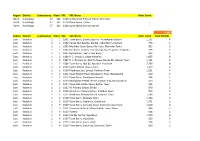
Region District Constituency Ward VRC VRC Name Voter Count North
Region District Constituency Ward VRC VRC Name Voter Count North Koinadugu 47 162 6169 Al-Harrakan Primary School, Woredala - North Koinadugu 47 162 6179 Open Space 2,Kabo - North Koinadugu 47 162 6180 Open Space, Kamayortortor - 9,493 Region District Constituency Ward VRC VRC Name Voter Count Total PS(100) East Kailahun 1 1 1001 Town Barry, Baoma, Baoma - Kunywahun Section 1,192 4 East Kailahun 1 1 1002 Palava Hut, Baoma, Baoma - Gborgborma Section 478 2 East Kailahun 1 1 1003 Mofindor Court Barry, Mofindor, Mofindor Town 835 3 East Kailahun 1 1 1004 Methodist primary school yengema, Yengama, Yengema 629 2 East Kailahun 1 1 1005 Nyanyahun Town, Town Barry 449 2 East Kailahun 1 2 1006 R. C. School 1, Upper Masanta 1,855 6 East Kailahun 1 2 1007 R. C. Primary 11, Gbomo Town, Buedu RD, Gbomo Town 1,121 4 East Kailahun 1 2 1008 Town Barry, Ngitibu, Ngitibu 1-Kailahum 2,209 8 East Kailahun 1 2 1009 KLDEC School, new London 1,259 4 East Kailahun 1 2 1010 Methodist Sec. School, Kailahun Town 1,031 4 East Kailahun 1 2 1011 Town Market Place, Bandajuma Town, Bandajuma 640 2 East Kailahun 1 2 1012 Town Barry, Bandajuma Sinneh 294 1 East Kailahun 1 2 1013 Bandajuma Health Centre, Luawa Foiya, Bandajuma Si 473 2 East Kailahun 1 2 1014 Town Hall, Borbu-Town, Borbu- Town 315 1 East Kailahun 1 2 1015 RC Primary School, Borbu 870 3 East Kailahun 1 2 1016 Amadiyya Primary School, Kailahun Town 973 3 East Kailahun 1 2 1017 Methodist Primary School, kailahun Town 1,266 4 East Kailahun 1 3 1018 Town Barry, Sandialu Town 1,260 4 East Kailahun 1 3 1019 Town -

Jan – Apr 2020 Activity Report
Rural Health Care Initiative Activity Report, January 2020 RHCI in Sierra Leone operates programs out of its office in Tikonko to support efforts in maternal and child health. The programs in this report include: ñ Mbao-mi Birth Waiting Home, Tikonko ñ Community Health Clinic birth waiting facility support, Kassama and Sembehun Tabema ñ Motorbike Outreach – Tikonko; serving Lembema, Dodo, Gbalehun and Sunga ñ Motorbike Outreach – Gondama; serving Gelehun, Sembehun Kokofele, Magbema and Gandorhun ñ Family Planning Services, Tikonko and surrounding villages Birth Waiting Homes The Mbao-mi accommodates 24 women and is staffed with a Senior Midwife, State Registered Nurse, Community Health Workers, security and administration personnel with two drivers for the RHCI vehicles. Women admitted are referred by the Tikonko Community Health Clinic (CHC) and receive lodging, food, health care, education, vocational training and transportation to the site of delivery and to their home village. Most women return to Mbao-mi after delivery for post-natal care, education and support. Mbao- mi provides staff and supplies to accompany the woman to the CHC for delivery. The Gondama Birth Waiting Home will accommodate 10 women and will provide similar services as Mbao-mi. The Kassama and Sembehun Tabema Peripheral Health Units (PHU’s) receive support from RHCI. The support includes medical supplies, medications and a stipend for food for women who stay at their facilities prior to labor and delivery. This arrangement was made between RHCI and these PHU’s due to their desire to keep deliveries at their CHC. Table 1. Number of Birth Waiting Home admissions in January, 2020. -

Table of Contents Table of Contents
1/2009 Delivering Justice to Sierra Leone’s Poor An Analysis of the Work of Timap for Justice Pamela Dale* * Comments and questions are welcome, and should be addressed to Pamela Dale ([email protected]). For questions on the World Bank‘s Justice for the Poor program in Sierra Leone, please contact Gibrill Jalloh ([email protected]), Lyttelton Braima ([email protected]), or [email protected]. DISCLAIMER Publications produced by the World Bank‘s Justice for the Poor program are intended to contribute to understanding, discussion, and debate on the practical and theoretical issues surrounding justice and governance reform. These publications provide the opportunity for a diverse array of authors to present interesting and up-to-date findings, tools, and lessons learned. Feedback from readers is encouraged, and should be sent to the author(s) at [email protected]. Though all J4P publications have undergone internal review to ensure factual accuracy and professional-quality research, the views expressed in these publications are those of the author(s), and do not necessarily reflect those of the World Bank, the Justice for the Poor program, or the program‘s funders and partners. Table of Contents Table of Contents ........................................................................................................................... i Acknowledgements ...................................................................................................................... iii Executive Summary .................................................................................................................... -

Establishment of a Genetically Confirmed Breeding Colony of Mastomys Natalensis from Wild-Caught Founders from West Africa
viruses Article Establishment of a Genetically Confirmed Breeding Colony of Mastomys natalensis from Wild-Caught Founders from West Africa David Safronetz 1,*,†, Kyle Rosenke 1, Robert J. Fischer 2,‡, Rachel A. LaCasse 3, Dana P. Scott 3, Greg Saturday 3, Patrick W. Hanley 3, Ousmane Maiga 4, Nafomon Sogoba 4, Tom G. Schwan 2 and Heinz Feldmann 1,* 1 Laboratory of Virology, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA; [email protected] 2 Laboratory of Zoonotic Pathogens, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA; fi[email protected] (R.J.F.); [email protected] (T.G.S.) 3 Rocky Mountain Veterinary Branch, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA; [email protected] (R.A.L.); [email protected] (D.P.S.); [email protected] (G.S.); [email protected] (P.W.H.) 4 International Center for Excellence in Research (ICER-Mali), Faculty of Medicine and Odonto Stomatology, University of Sciences, Techniques and Technologies of Bamako (USTTB), Bamako, Mali; [email protected] (O.M.); [email protected] (N.S.) * Correspondence: [email protected] (D.S.); [email protected] (H.F.) † Current address: Zoonotic Diseases and Special Pathogens, Public Health Agency of Canada, Winnipeg, MB R3E 3R2, Canada. Citation: Safronetz, D.; Rosenke, K.; ‡ Current Address: Laboratory of Virology, Rocky Mountain Laboratories, National Institute of Allergy Fischer, R.J.; LaCasse, R.A.; Scott, D.P.; and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA. -

California Legal Studies Journal Spring 2013
CALIFORNIA LEGAL STUDIES JOURNAL Editor-in-Chief Anna Cai Editors Carla Bernal Sun Kyu Park Business Manager Sun Kyu Park Cover Design ErineNatnat University of California, Berkeley Fall 2012—Spring2013 Copyright 2013 by California Legal Studies Journal Authors retain all rights to their articles. ASUC Sponsored California Legal Studies Journal is not an official publication of the Associated Students of the University of California. The views expressed herein are the views of the writers and not necessarily the views of the ASUC or the views of the University of California, Berkeley. Acknowledgements The publication of this journal would not have been possible without the following individuals: The Associated Students of the University of California Lauri la Pointe, Legal Studies Advisor. RominaFilippou, former editor. Colleen Lee, former editor-in-chief. The Berkeley legal Studies Association. Submission Information Paper Requirements: The paper can be of any length and any topic as long as it is law-related in some way. Neither you nor the class for which the paper was written must be in the Legal Studies department. We encourage students from all disciplines to submit papers, as the study of law itself is an interdisciplinary effort! Restrictions: We do not publish previously published works. You may submit your unpublished work to multiple journals. However, if your paper is accepted to another publication you must inform us immediately. What to submit: Your paper should be double-spaced. Please include the additional items: 1. Cover sheet with the following information: a. Full name. b. Class and term for which paper was written. c. -

Mastomys Spp. – Multimammate Mouse
Mastomys spp. – Multimammate Mouse Taxonomic status: Species Taxonomic notes: A good review of the systematics of Mastomys is provided by Granjon et al. (1997). Mastomys spp. are cryptic and difficult to distinguish morphologically but clearly separable by molecular and chromosomal markers (Britton-Davidian et al. 1995; Lecompte et al. 2005). For example, within the assessment region, M. coucha and M. natalensis can be distinguished only through chromosome number (in M. coucha 2n = 36; in M. natalensis 2n = 32) and molecular markers (Colangelo et al. 2013) but not on cranio-dental features, nor a multivariate analysis (Dippenaar et al. 1993). Mastomys coucha – Richard Yarnell Assessment Rationale Regional Red List status (2016) Both species are listed as Least Concern as they have a Mastomys coucha Least Concern wide distribution within the assessment region, where they likely occur in most protected areas, are abundant in Mastomys natalensis Least Concern human-transformed areas, including agricultural areas and areas affected by human disturbances, and because National Red List status (2004) there are no significant threats that could cause range- Mastomys coucha Least Concern wide decline. Additionally, these species are known as prolific breeders with population numbers likely to recover Mastomys natalensis Least Concern quickly after a decline. Because of their reproductive Reasons for change No change characteristics, population eruptions often occur under favourable conditions. Landowners and managers should Global Red List status (2016) pursue ecologically-based rodent management strategies Mastomys coucha Least Concern and biocontrol instead of rodenticides to regulate population explosions of this species. Mastomys natalensis Least Concern Regional population effects: For M. coucha, significant TOPS listing (NEMBA) (2007) None dispersal is unlikely because the bulk of the population CITES listing None occurs within the assessment region. -

Quaternary Murid Rodents of Timor Part I: New Material of Coryphomys Buehleri Schaub, 1937, and Description of a Second Species of the Genus
QUATERNARY MURID RODENTS OF TIMOR PART I: NEW MATERIAL OF CORYPHOMYS BUEHLERI SCHAUB, 1937, AND DESCRIPTION OF A SECOND SPECIES OF THE GENUS K. P. APLIN Australian National Wildlife Collection, CSIRO Division of Sustainable Ecosystems, Canberra and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) K. M. HELGEN Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution, Washington and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 341, 80 pp., 21 figures, 4 tables Issued July 21, 2010 Copyright E American Museum of Natural History 2010 ISSN 0003-0090 CONTENTS Abstract.......................................................... 3 Introduction . ...................................................... 3 The environmental context ........................................... 5 Materialsandmethods.............................................. 7 Systematics....................................................... 11 Coryphomys Schaub, 1937 ........................................... 11 Coryphomys buehleri Schaub, 1937 . ................................... 12 Extended description of Coryphomys buehleri............................ 12 Coryphomys musseri, sp.nov.......................................... 25 Description.................................................... 26 Coryphomys, sp.indet.............................................. 34 Discussion . .................................................... -

Payment of Tuition Fees to Primary Schools in Bo District for Second Term 2019/2020 School Year
PAYMENT OF TUITION FEES TO PRIMARY SCHOOLS IN BO DISTRICT FOR SECOND TERM 2019/2020 SCHOOL YEAR Amount NO. EMIS Name Of School Region District Chiefdom Address Headcount Total to School Per Child 1 311301222 Abdul Tawab Haikal Primary School South BO District Tikonko Samie 610 10000 6,100,000 Bo Kenema 2 319103274 Agape Way Christian Primary School South BO District Kakua 380 10000 Highway 3,800,000 3 311401201 Ahmadiyya Muslim Primary South BO District Valunia Baomahun 822 10000 8,220,000 4 310702210 Ahmadiyya Muslim Primary South BO District Jaima Koribondo 341 10000 3,410,000 5 310202206 Ahmadiyya Muslim Primary South BO District Bagbo Levuma 203 10000 2,030,000 Bumpe 6 310502209 Ahmadiyya Muslim Primary South BO District Makayoni 215 10000 Ngao 2,150,000 7 311401218 Ahmadiyya Muslim Primary South BO District Valunia Mandu 221 10000 2,210,000 8 310201205 Ahmadiyya Muslim Primary South BO District Bagbo Momajoe 338 10000 3,380,000 Bumpe 9 310503217 Ahmadiyya Muslim Primary South BO District Walihun 264 10000 Ngao 2,640,000 Baoma 10 310403210 Ahmadiyya Muslim Primary School South BO District Baoma 122 10000 Gbandi 1,220,000 Kenema 11 311401209 Ahmadiyya Muslim Primary School South BO District Valunia 330 10000 Blango 3,300,000 12 311001208 Ahmadiyya Muslim Primary School South BO District Lugbu Kpatobu 244 10000 2,440,000 13 310702215 Ahmadiyya Muslim Primary School South BO District Jaiama Kpetema 212 10000 2,120,000 14 310402205 Ahmadiyya Muslim Primary School South BO District Baoma Ndogbogoma 297 10000 2,970,000 15 310201211 Ahmadiyya -

Diversification of Muroid Rodents Driven by the Late Miocene Global Cooling Nelish Pradhan University of Vermont
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2018 Diversification Of Muroid Rodents Driven By The Late Miocene Global Cooling Nelish Pradhan University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Biochemistry, Biophysics, and Structural Biology Commons, Evolution Commons, and the Zoology Commons Recommended Citation Pradhan, Nelish, "Diversification Of Muroid Rodents Driven By The Late Miocene Global Cooling" (2018). Graduate College Dissertations and Theses. 907. https://scholarworks.uvm.edu/graddis/907 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. DIVERSIFICATION OF MUROID RODENTS DRIVEN BY THE LATE MIOCENE GLOBAL COOLING A Dissertation Presented by Nelish Pradhan to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Specializing in Biology May, 2018 Defense Date: January 8, 2018 Dissertation Examination Committee: C. William Kilpatrick, Ph.D., Advisor David S. Barrington, Ph.D., Chairperson Ingi Agnarsson, Ph.D. Lori Stevens, Ph.D. Sara I. Helms Cahan, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT Late Miocene, 8 to 6 million years ago (Ma), climatic changes brought about dramatic floral and faunal changes. Cooler and drier climates that prevailed in the Late Miocene led to expansion of grasslands and retreat of forests at a global scale. -

Sierra Leone Trial Monitoring Program Weekly Report
Page 1 of 4 U.C. Berkeley War Crimes Studies Center Sierra Leone Trial Monitoring Program Weekly Report Special Court Monitoring Program Update #49 Trial Chamber II - AFRC Trial Covering week ending July 15, 2005 by Michelle Staggs, Senior Researcher Summary Witness profiles Evidence at trial Legal and procedural issues Summary Proceedings in the AFRC trial continued to move quickly this week, with the prosecution calling a further eight witnesses in its case against the three accused. The accused each attended the trial for the majority of the week, the only exception being the absence of Santigie Borbor Kanu (aka “Five-five”) due to ill health. The Chamber closed on Friday so that members of the Sierra Leonean bar could attend the bar council elections. Witness profiles at a glance Witness TF1-033 is 45 years old and was born in Songo in the Port Loko District. The witness finished high-school and worked as a reporter for a local news tabloid, the State News Watch. The witness testified in English with the use of voice distortion. Witness TF1-055 is 75 years old and was born in Karina, in the Bombali district. The witness is married and has seven children currently alive. He testified in Madingo. Witness TF1-147 was born in Blama in the Bonthe district. He speaks Krio, Mende and English. He testified in Krio. Witness TF1-094 is a Category “C” witness (Victim of Sexual Violence) and was allegedly abducted as a child and a victim of multiple rape. She testified in Krio. Witness TF1-269 is a Category “C” witness (Victim of Sexual Violence) and testified with voice distortion.