The Control of Shoot Branching: an Example of Plant Information Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mothers in Science
The aim of this book is to illustrate, graphically, that it is perfectly possible to combine a successful and fulfilling career in research science with motherhood, and that there are no rules about how to do this. On each page you will find a timeline showing on one side, the career path of a research group leader in academic science, and on the other side, important events in her family life. Each contributor has also provided a brief text about their research and about how they have combined their career and family commitments. This project was funded by a Rosalind Franklin Award from the Royal Society 1 Foreword It is well known that women are under-represented in careers in These rules are part of a much wider mythology among scientists of science. In academia, considerable attention has been focused on the both genders at the PhD and post-doctoral stages in their careers. paucity of women at lecturer level, and the even more lamentable The myths bubble up from the combination of two aspects of the state of affairs at more senior levels. The academic career path has academic science environment. First, a quick look at the numbers a long apprenticeship. Typically there is an undergraduate degree, immediately shows that there are far fewer lectureship positions followed by a PhD, then some post-doctoral research contracts and than qualified candidates to fill them. Second, the mentors of early research fellowships, and then finally a more stable lectureship or career researchers are academic scientists who have successfully permanent research leader position, with promotion on up the made the transition to lectureships and beyond. -
BSCB Newsletter 2017D
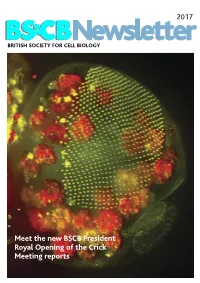
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

Female Fellows of the Royal Society
Female Fellows of the Royal Society Professor Jan Anderson FRS [1996] Professor Ruth Lynden-Bell FRS [2006] Professor Judith Armitage FRS [2013] Dr Mary Lyon FRS [1973] Professor Frances Ashcroft FMedSci FRS [1999] Professor Georgina Mace CBE FRS [2002] Professor Gillian Bates FMedSci FRS [2007] Professor Trudy Mackay FRS [2006] Professor Jean Beggs CBE FRS [1998] Professor Enid MacRobbie FRS [1991] Dame Jocelyn Bell Burnell DBE FRS [2003] Dr Philippa Marrack FMedSci FRS [1997] Dame Valerie Beral DBE FMedSci FRS [2006] Professor Dusa McDuff FRS [1994] Dr Mariann Bienz FMedSci FRS [2003] Professor Angela McLean FRS [2009] Professor Elizabeth Blackburn AC FRS [1992] Professor Anne Mills FMedSci FRS [2013] Professor Andrea Brand FMedSci FRS [2010] Professor Brenda Milner CC FRS [1979] Professor Eleanor Burbidge FRS [1964] Dr Anne O'Garra FMedSci FRS [2008] Professor Eleanor Campbell FRS [2010] Dame Bridget Ogilvie AC DBE FMedSci FRS [2003] Professor Doreen Cantrell FMedSci FRS [2011] Baroness Onora O'Neill * CBE FBA FMedSci FRS [2007] Professor Lorna Casselton CBE FRS [1999] Dame Linda Partridge DBE FMedSci FRS [1996] Professor Deborah Charlesworth FRS [2005] Dr Barbara Pearse FRS [1988] Professor Jennifer Clack FRS [2009] Professor Fiona Powrie FRS [2011] Professor Nicola Clayton FRS [2010] Professor Susan Rees FRS [2002] Professor Suzanne Cory AC FRS [1992] Professor Daniela Rhodes FRS [2007] Dame Kay Davies DBE FMedSci FRS [2003] Professor Elizabeth Robertson FRS [2003] Professor Caroline Dean OBE FRS [2004] Dame Carol Robinson DBE FMedSci -

Program Nr: 1 from the 2004 ASHG Annual Meeting Mutations in A
Program Nr: 1 from the 2004 ASHG Annual Meeting Mutations in a novel member of the chromodomain gene family cause CHARGE syndrome. L.E.L.M. Vissers1, C.M.A. van Ravenswaaij1, R. Admiraal2, J.A. Hurst3, B.B.A. de Vries1, I.M. Janssen1, W.A. van der Vliet1, E.H.L.P.G. Huys1, P.J. de Jong4, B.C.J. Hamel1, E.F.P.M. Schoenmakers1, H.G. Brunner1, A. Geurts van Kessel1, J.A. Veltman1. 1) Dept Human Genetics, UMC Nijmegen, Nijmegen, Netherlands; 2) Dept Otorhinolaryngology, UMC Nijmegen, Nijmegen, Netherlands; 3) Dept Clinical Genetics, The Churchill Hospital, Oxford, United Kingdom; 4) Children's Hospital Oakland Research Institute, BACPAC Resources, Oakland, CA. CHARGE association denotes the non-random occurrence of ocular coloboma, heart defects, choanal atresia, retarded growth and development, genital hypoplasia, ear anomalies and deafness (OMIM #214800). Almost all patients with CHARGE association are sporadic and its cause was unknown. We and others hypothesized that CHARGE association is due to a genomic microdeletion or to a mutation in a gene affecting early embryonic development. In this study array- based comparative genomic hybridization (array CGH) was used to screen patients with CHARGE association for submicroscopic DNA copy number alterations. De novo overlapping microdeletions in 8q12 were identified in two patients on a genome-wide 1 Mb resolution BAC array. A 2.3 Mb region of deletion overlap was defined using a tiling resolution chromosome 8 microarray. Sequence analysis of genes residing within this critical region revealed mutations in the CHD7 gene in 10 of the 17 CHARGE patients without microdeletions, including 7 heterozygous stop-codon mutations. -

Specification and Maintenance of the Floral Meristem: Interactions Between Positively- Acting Promoters of Flowering and Negative Regulators
SPECIAL SECTION: EMBRYOLOGY OF FLOWERING PLANTS Specification and maintenance of the floral meristem: interactions between positively- acting promoters of flowering and negative regulators Usha Vijayraghavan1,*, Kalika Prasad1 and Elliot Meyerowitz2,* 1Department of MCB, Indian Institute of Science, Bangalore 560 012, India 2Division of Biology 156–29, California Institute of Technology, Pasadena, CA 91125, USA meristems that give rise to modified leaves – whorls of A combination of environmental factors and endoge- nous cues trigger floral meristem initiation on the sterile organs (sepals and petals) and reproductive organs flanks of the shoot meristem. A plethora of regulatory (stamens and carpels). In this review we focus on mecha- genes have been implicated in this process. They func- nisms by which interactions between positive and nega- tion either as activators or as repressors of floral ini- tive regulators together pattern floral meristems in the tiation. This review describes the mode of their action model eudicot species A. thaliana. in a regulatory network that ensures the correct temporal and spatial control of floral meristem specification, its maintenance and determinate development. Maintenance of the shoot apical meristem and transitions in lateral meristem fate Keywords: Arabidopsis thaliana, floral activators, flo- ral mesitem specification, floral repressors. The maintenance of stem cells is brought about, at least in part, by a regulatory feedback loop between the ho- THE angiosperm embryo has a well established apical- meodomain transcription factor WUSCHEL (WUS) and basal/polar axis defined by the positions of the root and genes of the CLAVATA (CLV) signaling pathway2. WUS shoot meristems. Besides this, a basic radial pattern is is expressed in the organizing centre and confers a stem also established during embryogenesis. -

AXR1 Acts After Lateral Bud Formation to Inhibit Lateral Bud Growth in Arabidopsis
This is a repository copy of AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/262/ Article: Stirnberg, P., Leyser, O. and Chatfield, S.P. (1999) AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis. Plant Physiology. pp. 839-847. ISSN 0032-0889 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Plant Physiology, November 1999, Vol. 121, pp. 839–847, www.plantphysiol.org © 1999 American Society of Plant Physiologists AXR1 Acts after Lateral Bud Formation to Inhibit Lateral Bud Growth in Arabidopsis1 Petra Stirnberg, Steven P. Chatfield, and H.M. Ottoline Leyser* Department of Biology, University of York, P.O. Box 373, York YO10 5YW, United Kingdom Several mutants with altered auxin sensitivity have been The AXR1 gene of Arabidopsis is required for many auxin re- produced in Arabidopsis. -

Annual Report 2015
Annual Report 2015 Clare College Cambridge Contents Master’s Introduction .................................................................... 3 Teaching and Research .............................................................. 4–5 Selected Publications by Clare Fellows ....................................... 6–9 College Life ........................................................................... 10–12 Access & Outreach ..................................................................... 13 Financial Report ..................................................................... 14–15 Development ....................................................................... 16–17 List of Master & Fellows............................................................... 18 Captions ..................................................................................... 19 2 Master’s Introduction The past year has been full of many introductions for me two new CDs. They are fantastic ambassadors for the College and met some of our alumni in New as a relatively new Master - to student life in its various York - looking ahead; in 2016 they travel to Hong Kong, Malaysia and Singapore for more concerts. forms, to colleagues, and to our alumni at various events at home and abroad. The College continues to be in Last year I mentioned the need to refurbish Old Court, and this is still very much our priority. The good form, and weathering various new initiatives from Fellowship has just approved - in principle - plans which are now before Historic England, -

Signals Regulating Dormancy in Vegetative Buds
International Journal of Plant Developmental Biology ©2007 Global Science Books Signals Regulating Dormancy in Vegetative Buds Wun S. Chao* • Michael E. Foley • David P. Horvath • James V. Anderson Biosciences Research Laboratory, Plant Science Research, 1605 Albrecht Blvd., Fargo, ND 58105-5674, USA Corresponding author: * [email protected] ABSTRACT Dormancy in plants involves a temporary suspension of meristem growth, thus insuring bud survival and maintenance of proper shoot system architecture. Dormancy regulation is a complex process involving interactions of various signals through specific and/or overlapping signal transduction pathways. In this review, environmental, physiological, and developmental signals affecting dormancy are discussed. Environmental signals such as temperature and light play crucial roles in regulating development and release of bud dormancy. Physiological signals including phytochrome, phytohormones, and sugar are associated with changes in dormancy status that occur when plants perceive environmental signals. Developmental signals such as flowering and senescence also have an effect on bud dormancy. Currently, many genes and/or gene products are known to be responsive directly or indirectly to these signals. The potential roles for these genes in dormancy progression are discussed. _____________________________________________________________________________________________________________ Keywords: flowering, hormones, phytochrome, senescence, signaling, sugar, temperature Abbreviations: ABA, abscisic -

Inhibition of Lateral Shoot Formation by RNA Interference and Chemically Induced Mutations to Genes Expressed in the Axillary Meristem of Nicotiana Tabacum L
Hamano et al. BMC Plant Biol (2021) 21:236 https://doi.org/10.1186/s12870-021-03008-3 RESEARCH ARTICLE Open Access Inhibition of lateral shoot formation by RNA interference and chemically induced mutations to genes expressed in the axillary meristem of Nicotiana tabacum L. Kaori Hamano* , Seiki Sato, Masao Arai, Yuta Negishi, Takashi Nakamura, Tomoyuki Komatsu, Tsuyoshi Naragino and Shoichi Suzuki Abstract Background: Lateral branches vigorously proliferate in tobacco after the topping of the inforescence portions of stems for the maturation of the leaves to be harvested. Therefore, tobacco varieties with inhibited lateral shoot forma- tion are highly desired by tobacco farmers. Results: Genetic inhibition of lateral shoot formation was attempted in tobacco. Two groups of genes were exam- ined by RNA interference. The frst group comprised homologs of the genes mediating lateral shoot formation in other plants, whereas the second group included genes highly expressed in axillary bud primordial stages. Although “primary” lateral shoots that grew after the plants were topped of when fower buds emerged were unafected, the growth of “secondary” lateral shoots, which were detected on the abaxial side of the primary lateral shoot base, was signifcantly suppressed in the knock-down lines of NtLs, NtBl1, NtREV, VE7, and VE12. Chemically induced mutations to NtLs, NtBl1, and NtREV similarly inhibited the development of secondary and “tertiary” lateral shoots, but not primary lateral shoots. The mutations to NtLs and NtBl1 were incorporated into an elite variety by backcrossing. The agronomic characteristics of the backcross lines were examined in feld trials conducted in commercial tobacco production regions. The lines were generally suitable for tobacco leaf production and may be useful as new tobacco varieties. -

Genetic Assimilation and Canalisation in the Baldwin Effect
Genetic Assimilation and Canalisation in The Baldwin Effect Rob Mills and Richard A. Watson School of Electronics and Computer Science, University of Southampton, Southampton, UK, SO17 1BJ Email: [email protected], [email protected] Abstract. The Baldwin Effect indicates that individually learned behaviours acquired during an organism’s lifetime can influence the evolutionary path taken by a population, without any direct Lamarckian transfer of traits from phenotype to genotype. Several computational studies modelling this effect have included complications that restrict its applicability. Here we present a simplified model that is used to reveal the essential mechanisms and highlight several conceptual issues that have not been clearly defined in prior literature. In particular, we suggest that canalisation and genetic assimilation, often con- flated in previous studies, are separate concepts and the former is actually not required for non-heritable phenotypic variation to guide genetic variation. Ad- ditionally, learning, often considered to be essential for the Baldwin Effect, can be replaced with a more general phenotypic plasticity model. These simplifica- tions potentially permit the Baldwin Effect to operate in much more general cir- cumstances. 1 Introduction Our knowledge of modern genetics suggests that an organism's lifetime adaptations cannot influence the course of evolution because learned characteristics do not change ones own genes. In the late 19th century, Baldwin argued that although a direct effect of lifetime adaptation on genes is not possible, an indirect influence on the course of evolution is [1]. Subsequently his name has been associated with the impact that learning can have upon evolution. The ‘Baldwin Effect’ is based on two levels of search occurring: from generation to generation we have a slow genetic variation; and within each generation the variation due to lifetime learning is faster. -

Pruning Concepts; Apple
Winter Pruning for Best Results Dr. Ron Perry Department of Horticulture Michigan State University • Pruning: removal of a branch by mechanical means. • WHY: – Facilitate ease of cultural practices – Increase light penetration • Better fruit bud differentiation • Better fruit color & quality • Faster wound healing with UV light • Better flower bud development of flowering trees – Decrease mechanical injury to fruit & limbs • Limb rub • Fruit abrasion WHY: – Decrease fruit-bearing surface: better ratio of foliage/fruit • Better fruit size • Decrease fruit numbers • Decrease alternate bearing – Renew growth • Renews spur growth and vegetative growth – Maintain training system / structural framework – Remove broken, dying, & diseased branches – Better canopy air circulation= less disease, fruit cracking, better wound healing What happens to un-pruned trees? – Trees are Larger – Many branches; bush form (multi trunk) – Yield small fruits – Dense canopy – Bark inclusion – Poor structure / framework – More broken and diseased branches Plant Responses to Pruning • Pruning is a dwarfing process, that stimulates growth at localized sites ? From: Myers, S.C. and A. T. Savelle. 1996. Coordination of Vegetative and Reproductive Growth: Root restriction, branch manipulation and pruning. In, Tree Physiology Growth and Development, pub. by Good Fruit Grower, pp 69-80. Pruning……. • Removes usable reserves, both nitrogenous and carbohydrates. • Reduces next years’ growth potential • Loss of cambial /meristematic surface = net loss in wood growth • Growth near cuts is stimulated; overall, tree is reduced or dwarfed. • Therefore: pruning increases shoot growth but reduces total shoot and root growth. From: Forshey, C., D. Elfving and R. Stebbins. 1992. Training and Pruning Apple and Pear Trees. Pub. By Amer. Soc. Hort. Sci., 166 pages Plant Responses to Pruning Young Trees – Delays onset of fruit production in young trees – Reduces yields in early years – Strengthens framework scaffolding in young trees • Mature Trees – Reduces stored carbohydrates in wood. -

Environmental Variability and the Evolution of the Glucocorticoid Receptor (Nr3c1) in African Starlings
Ecology Letters, (2016) 19: 1219–1227 doi: 10.1111/ele.12656 LETTER Environmental variability and the evolution of the glucocorticoid receptor (Nr3c1) in African starlings Abstract Natalie R. Hofmeister1* and One of the primary ways that organisms cope with environmental change is through regulation of Dustin R. Rubenstein1,2 the hypothalamo–pituitary–adrenal (HPA) axis, the neuroendocrine system that controls reactions to stress. Variation in genes regulating the HPA axis – particularly the glucocorticoid receptor – may facilitate adaptation to changing climatic conditions by altering expression. Here we examine signatures of selection on the glucocorticoid receptor gene (Nr3c1) in African starlings that inhabit a range of environments, including those with variable climatic conditions. To investigate poten- tial adaptive mechanisms underlying the vertebrate stress response, we sequence the Nr3c1 gene in 27 species of African starlings. Although we find some evidence of positive selection, substitution rate is negatively correlated with variance in precipitation. This suggests climatic cycling in sub- Saharan Africa may have resulted in lower substitution rates to maintain a successful coping strategy. When environmental conditions fluctuate rapidly, variation in the strength of purifying selection can explain evolutionary rate variation. Keywords Adaptation, canalisation, environmental variability, fluctuating selection, stress response. Ecology Letters (2016) 19: 1219–1227 2015; Tufto 2015), we still do not fully understand how these INTRODUCTION phenotypic responses are governed at the genetic level. Climatic conditions that vary greatly in time can result in fluc- Climatic conditions in some environments may fluctuate too tuating selective pressures (Bell 2010). In an environment with unpredictably to allow for phenotypic plasticity or too rapidly extreme fluctuation, there is no single fitness function that to be tracked adaptively.