Hyaluronic Acid Functionalization with Jeffamine® M2005
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chloroform 18.08.2020.Pdf
Chloroform Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, sweet-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested. Formula: CHCl₃ IUPAC ID: Trichloromethane Molar mass: 119.38 g/mol Boiling point: 61.2 °C Density: 1.49 g/cm³ Melting point: -63.5 °C The molecule adopts a tetrahedral molecular geometry with C3v symmetry. Chloroform volatilizes readily from soil and surface water and undergoes degradation in air to produce phosgene, dichloromethane, formyl chloride, carbon monoxide, carbon dioxide, and hydrogen chloride. Its half-life in air ranges from 55 to 620 days. Biodegradation in water and soil is slow. Chloroform does not significantly bioaccumulate in aquatic organisms. Production:- In industry production, chloroform is produced by heating a mixture of chlorine and either chloromethane (CH3Cl) or methane (CH4). At 400–500 °C, a free radical halogenation occurs, converting these precursors to progressively more chlorinated compounds: CH4 + Cl2 → CH3Cl + HCl CH3Cl + Cl2 → CH2Cl2 + HCl CH2Cl2 + Cl2 → CHCl3 + HCl Chloroform undergoes further chlorination to yield carbon tetrachloride (CCl4): CHCl3 + Cl2 → CCl4 + HCl The output of this process is a mixture of the four chloromethanes (chloromethane, dichloromethane, chloroform, and carbon tetrachloride), which can then be separated by distillation. Chloroform may also be produced on a small scale via the haloform reaction between acetone and sodium hypochlorite: 3 NaClO + (CH3)2CO → CHCl3 + 2 NaOH + CH3COONa Deuterochloroform[ Deuterated chloroform is an isotopologue of chloroform with a single deuterium atom. -

Novel Reactions of Ceph-3-Ems
NOVEL REACTIONS OF CEPH-3-EMS Jacqueline M Torrance B.Sc. (Hons) A thesis submitted in partial fulfilment of the requirements of the University of Abertay Dundee for the degree of Doctor of Philosophy June 1995 I certify that this thesis is the true and accurate version of the thesis approved by the examiners. Signed Date (Director of Studies) DECLARATION I hereby declare that the work presented in this thesis was carried out by me at University of Abertay Dundee, Dundee, except where due acknowledgement is made, and has not been submitted by me for any other degree. Signed Date CONTENTS 1 INTRODUCTION 1 1.1 INTRODUCTION 2 1.1.1 History of Penicillin 2 1.1.2 History of Cephalosporins 4 1.2 REACTIONS AT SULPHUR 7 1.2.1 Oxidation 7 1.2.2 De-oxygenation 13 1.3 REACTIONS AT C-2 15 1.3.1 2-Alkylcephems 15 1.3.2 2-Alkoxycephems 17 1.3.3 2-Exocyclic Cephems 22 1.4 REACTIONS AT C-3 30 1.4.1 3-Exomethylene 30 1.4.2 3-Vinyl-and 3-Allylcephalosporins 38 1.4.3 3-Azidocephems 42 1.4.4 3-Alkoxy- and 3-Alkylcephems 44 1.5 REACTIONS AT C-4 46 1.5.1 A2-Cephems 46 1.5.2 Addition to the A3-Double Bond 51 1.5.3 Reactions at the C-4 Carboxyl Group 54 1.5.4 Ester Formation and Deprotection 59 1.6 REACTIONS AT C-7 62 1.6.1 7a-Methoxycephalosporins 62 1.6.2 7a-Formamidocephalosporins 66 1.6.3 Other 7a-Substituents 69 ii 1.6.4 7-Spirocyclic Cephalosporins 71 1.6.5 Reactions at N-7 73 1.7 MISCELLANEOUS CEPHALOSPORIN REACTIONS 74 1.7.1 Conversion of Penicillin to Cephalosporins 74 1.7.2 Conversion of Cephalosporins to Penicillins 78 1.7.3 Tricyclics and Tetracyclics -

Ep 3130580 A1
(19) TZZ¥_¥ZZ_T (11) EP 3 130 580 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 15.02.2017 Bulletin 2017/07 C07C 217/74 (2006.01) C07C 215/64 (2006.01) C07D 265/16 (2006.01) C07B 59/00 (2006.01) (2006.01) (21) Application number: 16183223.3 C07C 211/64 (22) Date of filing: 13.03.2008 (84) Designated Contracting States: • SEPEHR, Sarshar AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Vista, CA 92081-8356 (US) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT • WOO, Soon, Hyung RO SE SI SK TR Vista, CA 92081-8356 (US) (30) Priority: 15.03.2007 US 895049 P (74) Representative: Smaggasgale, Gillian Helen 15.06.2007 US 944399 P WP Thompson 138 Fetter Lane (62) Document number(s) of the earlier application(s) in London EC4A 1BT (GB) accordance with Art. 76 EPC: 08732084.2 / 2 125 698 Remarks: This application was filed on 08-08-2016 as a (71) Applicant: Auspex Pharmaceuticals, Inc. divisional application to the application mentioned Vista, CA 92081-8356 (US) under INID code 62. (72) Inventors: • GANT, Thomas G Vista, CA 92081-8356 (US) (54) PREPARATION OF DEUTERATED VENLAFAXINES (57) Processes for preparaing deuterated venlafaxines whcih can be used as inhibitors of the uptake of monoamine neurotransmitters. EP 3 130 580 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 130 580 A1 Description [0001] This application claims the benefit of priority of United States provisional applications No. -

A Study of the Anomalous Reaction of 4, 6-Dimethoxy-5-Nitropyrimidine With
AN ABSTRACT OF THE THESIS OF FRANK ARVID LEHMKUHL for the DOCTOR OF PHILOSOPHY (Name) (Degree) in CHEMISTRY presented on (Major) Title: A STUDY OF THE ANOMALOUS REACTION OF 4, 6- DIMETHOXY-5-NITROPYRIMMINE WITH METHYL- HYDRAZINE Abstract approved: Redacted for Privacy Bert E. Christensen The anomalous reaction of 4, 6-dimethoxy-5-nitropyrimidine with methylhydrazine in a pyridine solvent to yield 4-hydrazino-6-hydroxy- pyrimidine was investigated. When 4, 6- dimethoxy-5-nitropyrimidine was refluxed in pyridine in the absence of methylhydrazine two non- interconvertible salts were formed.The salt insoluble in cold pyri- dine had been previously identified as the methylpyridinium salt of 1, 6- dihydro- 4- hydr oxy- 1-methyl- 5-nitro- 6- oxopyr imi dine.The s olu- ble salt which reacts with methylhydrazine to yield 4-hydrazino-6- hydroxypyrimidine has been identified as the methylpyridinium salt of 4-hydroxy- -methoxy-5-nitropyrimidine.This salt reacts with the methylhydrazine as follows: H H ,,, OCH3 OC:3 CHNHNH - NH- NH2HV NiH 3 2 61-13 14;,%,,7'%NO2 H."*:;;%1 N HNFI © e CH3I G cH3 H HN, /4-HNH2 FIH I + %CH I I ,,,N\ 3 N,.."-OCHrl" -14 / H I I 60 3 I G 0 9 CH3 H NH2 CH3+CH3NHNH2 CH3 OH+ CHNHNH NH-NHCH3 3 N-OCH I e 3 0- 06 N- NH2 N- NH2 H H I I N-N- CH3 H ;OCH3 -CH3 Cna CH3 0I I N-NH2 H 9 /4-NCH. I N 3 FeS\JIL.,.."}; H CH3 H 0 H N-NH2 H + CH3 N=Ne CH? The tautomeric form resulting from the reaction pathway explains why the 5-proton does not appear in the nmr spectra of the compound when using either (a) sodium deuteroxide in deuterium oxide or (b) deuterium chloride in deuterium oxide solvents. -

WO 2018/023009 Al 01 February 2018 (01.02.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2018/023009 Al 01 February 2018 (01.02.2018) W !P O PCT (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, C07D 417/14 (2006.01) A61K 31/496 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, A61P 25/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, (21) International Application Number: KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, PCT/US20 17/044400 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 28 July 2017 (28.07.2017) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, 62/368,630 29 July 2016 (29.07.2016) US UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (71) Applicant: CONCERT PHARMACEUTICALS, INC. -

Table 4: Protective Action Criteria (PAC) Rev. 29 Based on Applicable
Table 4: Protective Action Criteria (PAC) Rev. 29a based on applicable 60-minute AEGLs, ERPGs, or TEELs. The chemicals are 3 listed in alphabetical order and the values are presented in mg/m . June 2018 Table 4 is an alphabetical list of the chemical substances and their corresponding PAC values in mass per unit volume (mg/m3). The conversion of ppm to mg/m3 was carried out assuming normal temperature and pressure, 25°C and 760 mm Hg. The columns presented in Table 4 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this alphabetical listing Chemical Name The name of the chemical substance submitted to the PAC development team CASRN The Chemical Abstracts Service Registry Number1 for this chemical PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value Chemicals for which AEGLs are available have their chemical name, CASRN, and AEGL values displayed in a bolded and larger font. Chemicals for which ERPGs are available, but not AEGLs, have their chemical name, CASRN, and ERPG values displayed in a bolded font. Chemicals for which TEELs are available, but no AEGLs or ERPGs, have their chemical name, CASRN, and values displayed using a regular font. Additional information on PAC values and TEEL values and links to other sources of information is provided on the Subcommittee on Consequence Assessment and Protective Actions (SCAPA) webpage at http://orise.orau.gov/emi/scapa/default.htm. -

Rev. 28A Table 3
Table 3: Protective Action Criteria (PAC) Rev. 28A based on applicable 60-minute AEGLs, ERPGs, or TEELs. The chemicals are listed by CASRN. February 2016 Table 3 presents a list of chemicals and associated PAC values in numerical order based on the CASRN1 for each chemical. Chemicals without an identified CASRN number are issued an identification number, preceded by the letter “z” for purposes of identification in the PAC data set. PACs are presented in either ppm or mg/m3. The columns presented in Table 3 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this listing by CASRN Chemical Name The name of the chemical substance submitted to the PAC development team CASRN The Chemical Abstracts Service Registry Number for this chemical PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value Units The units of the original request (ppm or mg/m3) Chemicals for which AEGLs are available have their chemical name, CASRN, and AEGL values displayed in a bolded and larger font. Chemicals for which ERPGs are available, but no AEGLs, have their chemical name, CASRN, and ERPG values displayed in a bolded font. Chemicals for which TEELs are available, but no AEGLs or ERPGs, have their chemical name, CASRN, and values displayed using a regular font. Additional information on PAC values, TEEL values, and links to other sources of information is provided on the Subcommittee on Consequence Assessment and Protective Actions (SCAPA) webpage at http://orise.orau.gov/emi/scapa/default.htm. -
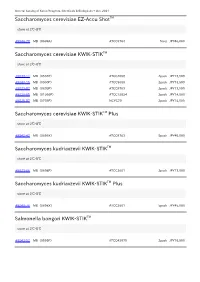
Saccharomyces Cerevisiae EZ-Accu Shottm
General Catalog of Kanto Reagents, Chemicals & Biologicals + Oct. 2021 Saccharomyces cerevisiae EZ-Accu ShotTM store at 2℃-8℃ 49944-78 MB(0699A) ATCC9763 5test JPY46,000 Saccharomyces cerevisiae KWIK-STIKTM store at 2℃-8℃ 49843-14 MB(0534P) ATCC4098 2pack JPY13,500 49843-15 MB(0900P) ATCC9080 2pack JPY13,500 49823-87 MB(0699P) ATCC9763 2pack JPY13,500 49820-65 MB(01066P) ATCC18824 2pack JPY14,500 49825-87 MB(0736P) NCYC79 2pack JPY14,500 Saccharomyces cerevisiae KWIK-STIKTM Plus store at 2℃-8℃ 49842-47 MB(0699X) ATCC9763 5pack JPY45,000 Saccharomyces kudriavzevii KWIK-STIKTM store at 2℃-8℃ 49823-86 MB(0698P) ATCC2601 2pack JPY13,500 Saccharomyces kudriavzevii KWIK-STIKTM Plus store at 2℃-8℃ 49840-76 MB(0698X) ATCC2601 5pack JPY45,000 Salmonella bongori KWIK-STIKTM store at 2℃-8℃ 49843-52 MB(0595P) ATCC43975 2pack JPY16,500 General Catalog of Kanto Reagents, Chemicals & Biologicals + Oct. 2021 Salmonella enterica subsp.arizonae EZ-Accu ShotTM store at 2℃-8℃ 49946-13 MB(0901A) ATCC13314 5test JPY46,000 Salmonella enterica subsp.arizonae KWIK-STIKTM store at 2℃-8℃ 49823-91 MB(0901P) ATCC13314 2pack JPY13,500 Salmonella enterica subsp.diarizonae KWIK-STIKTM store at 2℃-8℃ 49820-33 MB(01054P) ATCC12325 2pack JPY14,500 49844-90 MB(01045P) ATCC29934 2pack JPY14,500 Salmonella enterica subsp.enterica serotype Abony EZ-CFUTM store at 2℃-8℃ 49840-79 MB(0890C) NCTC6017 10test JPY87,000 Salmonella enterica subsp.enterica serotype Abony EZ-CFUTM One Step store at 2℃-8℃ 49842-70 MB(0890Z) NCTC6017 10test JPY86,000 Salmonella enterica subsp.enterica serotype Abony KWIK-STIKTM store at 2℃-8℃ 49825-88 MB(0890P) NCTC6017 2pack JPY11,500 General Catalog of Kanto Reagents, Chemicals & Biologicals + Oct. -

Mechanism of Reaction of Carbon Monoxide with Phenyllithium'
lReprintedfrom the Journalof the AmericanChemical Society, 95,8118 (1973).] Copyright 1973by the American Chemical Societyand reprinted by permissionof the copyright owner. Mechanism of Reaction of Carbon Monoxide with Phenyllithium' Larry S. Trzupek, Terry L. Newirth,2 Edward G. Kelly, Norma Ethyl Sbarbati,sand George M. Whitesides* Contribution from the Department of Chemistry, MassachusettsInstitute of Technology,Cambridge, Massachusetts 02139. ReceiuedJuly 14, 1973 Abstract: The product mixtures obtained by reaction between phenyllithium and carbon monoxide in diethyl ether, followed by hydrolysis, include benzophenone (1). a,a-diphenylacetophenone(2), benzil (3), a,a-diphenyl- a-hydroxyacetophenone (4), benzpinacot (5), a-hydroxyacetophenone (6), 1,3,3-triphenylpropane-1,2-dione(7), 1,3,3-triphenylpropan-1-one-2,3-diol(8). and benzhydrol (9). Compounds 1, 2, 6,7, and 8 are produced in signifi- cant yields i 3,4,5, and 9 are produced in trace quantities. Spectroscopicstudies establish dilithium benzophenone dianion (18) as the first long-lived intermediate formed in this reaction; qualitative correlations betweenthe basicity of a number of organolithium reagentsand their reactivity toward carbon monoxide suggests,but does not prove, that benzoyllithium is a precursor of 18. Labeling experiments indicate that the products ultimately isolated following hydrolysis of the reaction mixture are derived from at least two pathways which compete for the initially formed 18. One involves combination of l8 with 1 equiv of phenyllithium and 1 equiv of carbon monoxide, followed by elimination of I equiv of lithium oxide. yielding 17, the lithium enolateof 2; a secondinvolves com- bination of 18 with 1 equiv of phenyllithium and 2 equiv of carbon monoxide. -

Deuterated Solvents, Reagents & Accessories for NMR and Synthetic Applications
isotope.com Deuterated Solvents, Reagents & Accessories for NMR and Synthetic Applications • NMR Solvents • NMR Reference Standards • NMR Tubes • qNMR • NMR Protein Standards • Deuterated Detergents • Deuterated Buffers • Synthetic Intermediates Enriching Scientific Discovery Ordering Information The CIL Customer Service Department is open from 8:00 a.m. to 5:30 p.m. Eastern Standard Time. Orders may be placed by fax, email or via our website 24 hours a day. Phone: 1.800.322.1174 (North America) 1.978.749.8000 Fax: 1.978.749.2768 Email: [email protected] [email protected] (International) Website: isotope.com isotope.com TABLE OF CONTENTS Table of Contents Corporate Overview . 2 NMR Solvents and Deuterated Reagents Joel Bradley, PhD . 3 Quality Control Spotlight Tim Eckersley, PhD . 3 Cambridge Isotope Laboratories, Inc . Facilities . 4 Ordering and Contact Information . 6 Shipping Information / Product Information . 7 What Is an Isotope? . 8 Isotopic Enrichment . 8 Globally Harmonized System (GHS) of Classification and Labeling of Chemicals . 9 Understanding the Product Listings . 10 CIL Labels . 10 NMR Analysis . 11-26 NMR Product Listing . 12 NMR Reference Standards . 19 NORELL® Tubes . 20 Wilmad-LabGlass NMR Sample Tubes . 21 Use and Handling of NMR Solvents . 22 NMR Solvent Technical Tips . 22 Deuterated Chloroform . 23 NMR Solvent Data Chart . 24 NMR Solvent Storage and Handling Information . 25 NMR Solvents Chemical Shifts of Selected Compounds in Different Solvents . 26 qNMR . 27-29 Standards for qNMR . 28 Synthesis . 31-35 Stable Isotope-Labeled Synthetic Intermediates . 32 Getting Started with Using Deuterated Products? Or Looking for New Ideas? . 33 New Methods for Quantitation of Environmental Pollutants . 35 Biomolecular NMR . -

Trihydrate, 99.9% (Metals Basis), Pt 45.2% Potassium Tetracyano
Product 유해물질명 010536 Potassium tetracyanoplatinate(II) trihydrate, 99.9% (metals basis), Pt 45.2% Potassium tetracyanoplatinate(II) trihydrate 010661 Cadmium chloride hydrate, Puratronic®, 99.998% (metals basis) Cadmium chloride hydrate 011138 Zinc sulfate hydrate, Puratronic®, 99.999% (metals basis) Zinc sulfate hydrate 011865 Cadmium acetate dihydrate, 99.999% (metals basis) Cadmium acetate dihydrate 012373 Cadmium chloride hemipentahydrate, ACS, 79.5-81.0% Cadmium chloride hemipentahydrate 012514 Nickel(II) sulfate hexahydrate, 98% Nickel(II) sulfate hexahydrate 012611 Sodium dichromate dihydrate, Reagent Grade Sodium dichromate dihydrate 014127 Zinc fluoride tetrahydrate, 98% Zinc fluoride tetrahydrate 014160 Zinc sulfate monohydrate, Zn 35.5% Zinc sulfate monohydrate 017553 Lead(II) trifluoroacetate hemihydrate Lead(II) trifluoroacetate hemihydrate 032958 Cadmium bromide tetrahydrate, Reagent Grade Cadmium bromide tetrahydrate 033373 Sodium hydrogen arsenate heptahydrate, ACS, 98.0-102.0% Sodium hydrogen arsenate heptahydrate 040503 Magnesium chromate hydrate, 99.8% (metals basis) Magnesium chromate hydrate 044230 Zinc bromide hydrate, 99.9% (metals basis) Zinc bromide hydrate 044315 Zinc perchlorate hexahydrate, 99.999% (metals basis) Zinc perchlorate hexahydrate 053108 Nickel tetrafluoroborate hexahydrate Nickel tetrafluoroborate hexahydrate 053130 Nickel(II) sulfate hexahydrate, 99.97% min (metals basis) Nickel(II) sulfate hexahydrate 087683 Sodium pentacyanonitrosylferrate(III) dihydrate, ACS, 99.0-102.0% Sodium pentacyanonitrosylferrate(III) -

The Chemistry of 6-Hydroxycrinamine and Related Compounds Charles Franklin Murphy Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1966 The chemistry of 6-hydroxycrinamine and related compounds Charles Franklin Murphy Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Murphy, Charles Franklin, "The chemistry of 6-hydroxycrinamine and related compounds " (1966). Retrospective Theses and Dissertations. 5330. https://lib.dr.iastate.edu/rtd/5330 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfihned exactly as received ®7-2088 MURPHY, Charles Franklin, 1940- THE CHEMISTRY OF 6-HYDROXYCRINAMINE AND RELATED COMPOUNDS. Iowa State University of Science and Technology, Ph.D., 1966 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan THE CHEMISTRY OF 6-HYDROXYCRINAMINE AND RELATED COMPOUNDS BY CHARLES FRANKLIN MURPHY A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY MAJOR SUBJECTI ORGANIC CHEMISTRY APPROVED* Signature was redacted for privacy. IN CHARGE OF MAJOR WORK Signature was redacted for privacy. HEAD LOF MAJOR DEPARTMENT