Current NPS Threats Volume III October 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DEPARTMENT of JUSTICE Drug Enforcement
This document is scheduled to be published in the Federal Register on 08/03/2021 and available online at DEPARTMENT OF JUSTICEfederalregister.gov/d/2021-16499, and on govinfo.gov Drug Enforcement Administration Bulk Manufacturer of Controlled Substances Application: Cerilliant Corporation [Docket No. DEA-873] AGENCY: Drug Enforcement Administration, Justice. ACTION: Notice of application. SUMMARY: Cerilliant Corporation has applied to be registered as a bulk manufacturer of basic class(es) of controlled substance(s). Refer to Supplemental Information listed below for further drug information. DATES: Registered bulk manufacturers of the affected basic class(es), and applicants therefore, may file written comments on or objections to the issuance of the proposed registration on or before [INSERT DATE 60 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER]. Such persons may also file a written request for a hearing on the application on or before [INSERT DATE 60 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER]. ADDRESS: Written comments should be sent to: Drug Enforcement Administration, Attention: DEA Federal Register Representative/DPW, 8701 Morrissette Drive, Springfield, Virginia 22152. SUPPLEMENTARY INFORMATION: In accordance with 21 CFR 1301.33(a), this is notice that on June, 24, 2021, Cerilliant Corporation, 811 Paloma Drive, Suite A, Round Rock, Texas 78665-2402, applied to be registered as a bulk manufacturer of the following basic class(es) of controlled substance(s): Controlled Substance Drug Code Schedule 3-Fluoro-N-methylcathinone -

Toxicology Report Division of Toxicology Daniel D
Franklin County Forensic Science Center Office of the Coroner Anahi M. Ortiz, M.D. 2090 Frank Road Columbus, Ohio 43223 Toxicology Report Division of Toxicology Daniel D. Baker, Chief Toxicologist Casey Goodson Case # LAB-20-5315 Date report completed: January 28, 2021 A systematic toxicological analysis has been performed and the following agents were detected. Postmortem Blood: Gray Top Thoracic ELISA Screen Acetaminophen Not Detected ELISA Screen Barbiturates Not Detected ELISA Screen Benzodiazepines Not Detected ELISA Screen Benzoylecgonine Not Detected ELISA Screen Buprenorphine Not Detected ELISA Screen Cannabinoids See Confirmation ELISA Screen Fentanyl Not Detected ELISA Screen Methamphetamine Not Detected ELISA Screen Naltrexone/Naloxone Not Detected ELISA Screen Opiates Not Detected ELISA Screen Oxycodone/Oxymorphone Not Detected ELISA Screen Salicylates Not Detected ELISA Screen Tricyclics Not Detected Page 1 of 4 Casey Goodson Case # LAB-20-5315 GC/FID Ethanol Not Detected GC/MS Acidic/Neutral Drugs None Detected GC/MS Nicotine Positive GC/MS Cotinine Positive Reference Lab Delta-9-THC 13 ng/mL Reference Lab 11-Hydroxy-Delta-9-THC 1.2 ng/mL Reference Lab 11-Nor-9-Carboxy-Delta-9-THC 15 ng/mL Postmortem Urine: Gray Top Urine GC/MS Cotinine Positive This report has been verified as accurate and complete by ______________________________________ Daniel D. Baker, M.S., F-ABFT Cannabinoid quantitations in blood were performed by NMS Labs, Horsham, PA. Page 2 of 4 Casey Goodson Case # LAB-20-5315 Postmortem Toxicology Scope of Analysis Franklin County Coroner’s Office Division of Toxicology Enzyme Linked Immunosorbant Assay (ELISA) Blood Screen: Qualitative Presumptive Compounds/Classes: Acetaminophen (cut-off 10 µg/mL), Benzodiazepines (cut-off 20 ng/mL), Benzoylecgonine (cut-off 50 ng/mL), Cannabinoids (cut-off 40 ng/mL), Fentanyl (cut-off 1 ng/mL), Methamphetamine/MDMA (cut-off 50 ng/mL), Opiates (cut-off 40 ng/mL), Oxycodone/Oxymorphone (cut-off 40 ng/mL), Salicylates (50 µg/mL). -

(SEOW) Meeting Minutes, October 21, 2020
MINUTE RECORD MICHIGAN DEPARTMENT OF HEALTH AND HUMAN SERVICES (MDHHS) OFFICE OF RECOVERY ORIENTED SYSTEMS OF CARE (OROSC) State Epidemiological Outcomes Workgroup (SEOW) WORKGROUP NAME State Epidemiological Outcomes Workgroup (SEOW) Core Team DATE Wednesday, October 21, 2020 TIME 1:00 p.m. – 2:00 p.m. LOCATION Virtual Meeting CHAIRPERSON Joel Hoepfner RECORDER BlueJeans System ATTENDEES PHONE: Prashanti Boinapally, Lisa Coleman, Jane Goerge, Choua Gonzalez- Medina, Alicia Goodman, David Havens, Joel Hoepfner, Brandon Hool, Rich Isaacson, Rita Seith, Thomas Largo, Stephanie Larocco, Patricia McKane, Mary Miller, Janelle Murray, Hannah Napolillo, Su Min Oh, Dawn Radzioch, Brooke Rodriguez, Samantha Jones, Christy Sanborn, Larry Scott, Gabrielle Stroh-Steiner ABSENT: Liz Agius, Bret Bielawski, Sandra Bullard, Joe Coyle, Seth Eckle, Kimberly Fox, Brian Hartl, Denise Herbert, Patrick Hindman, Rachel Jantz, Jeanne Kapenga, Haley Kehus, Scott Josephs, Alia Lucas, Mary Ludtke, Jamie Meister, Logan O’Neill, Annette Perrino, Eva Petoskey, Sarah Rockhill, Kelsey Schell, Angie Smith-Butterwick, Ricki Torsch, Stephanie VanDerKooi, Danielle Walsh SUMMARY OF KEY POINTS Introductions, Welcome, and Approval of Agenda/Minutes – Su Min Oh Agenda reviewed and added heroin emerging issues and trends is now approved. If anyone has any items for future agendas, please contact Larry Scott ([email protected]), Angie Smith-Butterwick ([email protected]), Su Min Oh ([email protected]), Lisa Coleman ([email protected], or Joel Hoepfner at [email protected]. -

Metonitazene Begins Proliferation As Newest Synthetic Opioid Among Latest Cycle of Non-Fentanyl Related Drugs
January 2021 Metonitazene Begins Proliferation as Newest Synthetic Opioid Among Latest Cycle of Non-Fentanyl Related Drugs Purpose: The objective of this announcement is to notify public health and safety, law enforcement, first responders, clinicians, medical examiners and coroners, forensic and clinical laboratory personnel, and all other related Demographics communities about new information surrounding the emergent synthetic opioid metonitazene. Case Type: • Postmortem (n=8) Background: Synthetic opioids are chemically manufactured drugs, often accompanied with unknown potency and adverse effects or health risks. New synthetic opioids may be mixed with more traditional opioids, creating additional Age: • Range: 30s to 50s risk and danger for recreational drug users. Synthetic opioids may be distributed in powder or tablet form. In the United States (U.S.), an alarming increase in the number of deaths linked to synthetic opioid use has been reported. Date of Collection: • Aug. to Dec. 2020 The primary adverse effect associated with synthetic opioid use is respiratory depression, often leading to death. Other Notable Findings: Summary: Metonitazene is a potent synthetic opioid bearing structural resemblance to etonitazene, a synthetic opioid • Fentanyl (n=6) that is nationally and internationally controlled. Metonitazene is dissimilar in structure to other synthetic opioids • Cocaine (n=4) typically encountered in forensic casework (e.g. fentanyl analogues). Metonitazene and similar analogues (e.g. • Methamphetamine (n=4) etonitazene, isotonitazene) were first synthesized and reported in the literature in the 1950s. Pharmacological data suggest that this group of synthetic opioids have potency similar to or greater than fentanyl. Metonitazene was first reported by NPS Discovery after detection in a seized drug powder in July 2020. -

NFLIS-Drug Selected Substance List
2017-2020 NFLIS-Drug Substance List (Sorted by Date) Date Added NFLIS Substance Name Synonyms Chemical Name Structure InChI Formula to NFLIS- Drug InChI=1S/C16H20BrN/ c17-14-1-3-15(4-2-14)18-16-12-6-10-5-11 Bromantane ladasten N-(4-bromophenyl)adamantan-2-amine C16H20BrN 12/7/20 (8-12)9-13(16)7-10/h1-4,10-13,16,18H, 5-9H2 InChI=1S/C21H29FN2O3/ c1-4-27-21(26)19(15(2)3)23-20(25)17-14- ethyl 2-(1-(5-fluoropentyl)-1H-indole-3-carboxamido)-3- 5F-EMB-PICA EMB-2201; 5-fluoro-EMB-PICA 24(13-9-5-8-12-22)18-11-7-6-10-16(17)18 C21H29FN2O3 11/12/20 methylbutanoate /h6-7,10-11,14-15,19H, 4-5,8-9,12-13H2,1-3H3,(H,23,25) InChI=1S/C20H27FN2O3/ c1-20(2,3)17(19(25)26-4)22-18(24)15-13- methyl 2-(1-(4-fluorobutyl)-1H-indole-3- 4F-MDMB-BUTICA 4-fluoro-MDMB-BUTICA; 4F-MDMB-BICA 23(12-8-7-11-21)16-10-6-5-9-14(15)16/ C20H27FN2O3 10/23/20 carboxamido)-3,3-dimethylbutanoate h5-6,9-10,13,17H,7-8,11-12H2,1-4H3,(H, 22,24) InChI=1S/C10H14BrNO2/ 4-methoxy-6-[(1E)-2-phenylethenyl]-5,6-dihydro-2H- 2Br-4,5-Dimethoxyphenethylamine 2-bromo-4,5-dimethoxyphenethylamine c1-13-9-5-7(3-4-12)8(11)6-10(9)14-2/ C10H14BrNO2 10/2/20 pyran-2-one h5-6H,3-4,12H2,1-2H3 InChI=1S/C16H22FNO/ 4-fluoro-3-methyl-alpha-PVP; 4F-3-methyl-alpha- c1-3-6-15(18-9-4-5-10-18)16(19)13-7-8-1 4F-3-Methyl-alpha-PVP 4-fluoro-3-methyl-alpha-pyrrolidinopentiophenone C16H22FNO 10/2/20 pyrrolidinovalerophenone 4(17)12(2)11-13/h7-8,11,15H, 3-6,9-10H2,1-2H3 InChI=1S/C21H26N4O3/ N,N-diethyl-2-[2-(4-methoxybenzyl)-5-nitro-1H- c1-4-23(5-2)12-13-24-20-11-8-17(25(26)2 Metonitazene C21H26N4O3 9/15/20 benzimidazol-1-yl]ethanamine -

Phar-Mon Plus
Phar-Mon plus Der Konsum etablierter sowie neuer psychoaktiver Substanzen in unterschiedlichen Risikopopulationen Ergebnisse des Projekts Phar-Mon plus aus dem Jahr 2020 Dr. Kirsten Lochbühler, Regina Kühnl, Simona Maspero, Darya Aydin & Mark Hulm IFT Institut für Therapieforschung DANKSAGUNG Unser herzlicher Dank gilt denjenigen, die uns bei der Erstellung des Berichts unterstützt haben: unserem Kooperationspartner Karsten Tögel-Lins von Basis e. V., unseren Kolleginnen Renate Schlüter, Julia Heck, Monika Rossa, Katharina Schoder und Selina Moser sowie unseren Praktikantinnen und Praktikanten Helena Faust, Elena Schauer, Anna Hodges, Olivia Hoppe, Jan Kustermann, Anna Preitenwieser, Tim Sedelmaier, Lisa Pfefferseder und Magdalena Wimmer. Darüber hinaus möchten wir all unseren engagierten Kooperationspartnerinnen und -partnern danken: den Partyprojekten, den Suchthilfeeinrichtungen, dem HaLT-Projekt der BAS, dem GIZ- Nord und der JVA Wittlich, die uns seit Jahren bei der Rekrutierung von Studienteilnehmerinnen und -teilnehmern unterstützen bzw. uns Daten aus ihren Projekten/Einrichtungen zur Verfügung stellen. Außerdem möchten wir uns bei allen Personen, die sich für ein Interview bereit erklärt haben, ganz herzlich bedanken. Ein besonderer Dank geht auch an alle Teilnehmerinnen und Teilnehmer, die Fragebögen ausgefüllt haben und uns somit einen wertvollen Einblick in ihr Konsumverhalten erlauben. 3 INHALT Inhalt Teil 1: Auswirkungen der COVID-19-Pandemie auf den Konsum psychoaktiver Substanzen und das Suchthilfesystem ............................................................ -

National Forensic Laboratory Information System: NFLIS-Drug
snapshot NATIONAL FORENSIC LABORATORY INFORMATION SYSTEM DRUG March 2021 Newly Reported Drugs: The following drugs were reported to NFLIS-Drug for the first time between January 1, 2021, and March 31, 2021. South Midwest Northeast MDMB-4en-PICA ADB-BINACA para-Chlorofentanyl 4F-ABUTINACA AP-238 5F-EDMB-PICA Snapshot of Drug Reports Cannabinoids 34,492 Synthetic Cannabinoids 2,911 Phenethylamines 86,891 Received by NFLIS-Drug Cannabis/THC 33,087 MDMB-4en-PINACA 1,297 Methamphetamine 82,984 Cannabidiol 813 Fluoro-MDMB-PICA1 438 Amphetamine 1,590 The tables on the right Cannabinol 211 Fluoro-MDMB-BUTINACA1 182 MDMA 1,079 present the top five drug Tetrahydrocannabinolic acid A 204 ADB-BUTINACA 112 MDA 167 reports in each category delta-8-Tetrahydrocannabinol 98 Fluoro-MDMB-BUTICA1 101 Lisdexamfetamine 131 received by NFLIS-Drug between January 1, 2021, Selected Selected Synthetic Selected Narcotic and March 31, 2021. Benzodiazepines 7,956 Cathinones 2,809 Analgesics 42,384 Etizolam 1,161 Eutylone 2,381 Phenyl fentanyl 151 Flualprazolam 743 BMDP 81 Fluorofentanyl1 77 Clonazolam 692 N-Ethylpentylone 54 Brorphine 28 1 Includes all positional and nonspecified isomers Flubromazolam 142 3,4-Methylenedioxy PV8 45 Isotonitazene 26 as reported by participating laboratories. Diclazepam 53 Fluoro-methyl-alpha-PVP1 17 Metonitazene 23 Percentage of All NFLIS-Drug Items Containing Fentanyl and at Least One Other Drug2 2020 2019 2018 Heroin 60% Heroin 67% Heroin 77% Tramadol 14% Tramadol 8% Tramadol 9% ANPP 14% ANPP 10% ANPP 3% Cocaine 7% Cocaine 8% Cocaine 8% Acetyl fentanyl 7% Acetyl fentanyl 21% Acetyl fentanyl 14% Methamphetamine 7% Methamphetamine 5% Methamphetamine 3% Xylazine 6% Xylazine 3% Xylazine 1% Acetaminophen 5% Acetaminophen 3% Acetaminophen 2% 0% 20% 40% 60% 80% 100% 0% 20% 40% 60% 80% 100% 0% 20% 40% 60% 80% 100% 2 The data presented in these figures do not necessarily reflect true combinations (e.g., powders mixed together) but also include instances of separate drugs reported together in the same item. -
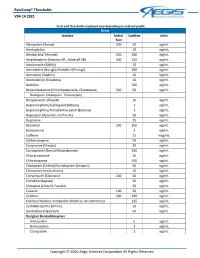
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

Brorphine — a Potent New Synthetic Opioid Identified in the Midwestern United States
July 2020 The Rise of Brorphine — A Potent New Synthetic Opioid Identified in the Midwestern United States Purpose: The objective of this announcement is to notify public health and safety, law enforcement, first responders, clinicians, medical examiners and coroners, forensic and clinical laboratory personnel, and all other related Brorphine Related Deaths communities about new information surrounding the emergent synthetic opioid brorphine. Demographics Age: Background: Synthetic opioids are chemically manufactured drugs, often accompanied with unknown potency and • Avg. 52, Med. 53 adverse effects or health risks. New synthetic opioids may be mixed with more traditional opioids, creating additional • Range: 40’s to 60’s risk and danger for recreational drug users. Synthetic opioids may be distributed in powder or tablet form. In the United States (U.S.), an alarming increase in the number of deaths linked to synthetic opioid use has been reported. Sex: The primary adverse effect associated with synthetic opioid use is respiratory depression, often leading to death. • Male (n=6), Female (n=1) Summary: Our laboratory previously reported the appearance of the potent synthetic opioid isotonitazene on the Case Type: illicit drug market in late 2019. Data from controlled substance testing showed drug materials containing • Postmortem (n=7) isotonitazene appeared as gray granular powder, often in combination with flualprazolam, an illicit benzodiazepine. Specimen Type: In June 2020, the U.S. Drug Enforcement Administration (DEA) temporarily scheduled isotonitazene. Shortly there • Blood (n=7) after, detections of brorphine in the U.S. began to increase, appearing as similar gray drug powders and an apparent Date of Collection: replacement for isotonitazene. -

21.8018.02000 FIRST ENGROSSMENT Sixty-Seventh Legislative Assembly ENGROSSED SENATE BILL NO
21.8018.02000 FIRST ENGROSSMENT Sixty-seventh Legislative Assembly ENGROSSED SENATE BILL NO. 2059 of North Dakota Introduced by Judiciary Committee (At the request of the State Board of Pharmacy) 1 A BILL for an Act to amend and reenact subsection 18 of section 19-03.1-01 and sections 2 19-03.1-05, 19-03.1-07, 19-03.1-11 and 19-03.1-13 of the North Dakota Century Code, relating 3 to the definition of marijuana and the scheduling of controlled substances; and to declare an 4 emergency. 5 BE IT ENACTED BY THE LEGISLATIVE ASSEMBLY OF NORTH DAKOTA: 6 SECTION 1. AMENDMENT. Subsection 18 of section 19-03.1-01 of the North Dakota 7 Century Code is amended and reenacted as follows: 8 18. "Marijuana" means all parts of the plant cannabis sativa L., whether growing or not; 9 the seeds thereof; the resin extracted from any part of the plant; and every compound, 10 manufacture, salt, derivative, mixture, or preparation of the plant, its seeds, or resin. 11 The term does not include the mature stalks of the plant, fiber produced from the 12 stalks, oil or cake made from the seeds of the plant, any other compound, 13 manufacture, salt, derivative, mixture, or preparation of mature stalks, except the resin 14 extracted therefrom, fiber, oil, or cake, or the sterilized seed of the plant which is 15 incapable of germination. The term marijuana does not include hemp as defined in 16 title 4.1means all parts of the plant cannabis sativa L., whether growing or not; the 17 seeds thereof; the resin extracted from any part of the plant; and every compound, 18 manufacture, salt, derivative, mixture, or preparation of the plant, its seeds, or resin. -

Eu Early Warning System Situation Report
EU EARLY WARNING SYSTEM SITUATION REPORT Situation report 1 — June 2020 SITREP ID: EU-EWS-SITREP-2020-0001 Issued by: EMCDDA Date issued: 16/06/2020 Transmitted by: Action on New Drugs Sector, EMCDDA Recipients: Early Warning System Network Contents 1. Purpose 2 2. General update of the NPS situation 2 3. Operational matters 7 4. Subject focus: Potential impact of the COVID-19 pandemic on the drug markets and risks to people who use drugs 8 5. Update from the national early-warning systems 11 6. Publications and resources of interest 11 7. New psychoactive substances notified in 2020 — provisional list, 1 January–16 June 2020 13 8. References 15 EU-EWS-SITREP-2020-0001 1 of 16 1. Purpose The purpose of this situation report is to: provide a summary of recent information reported to the EU Early Warning System, including formal notifications and other important signals; highlight important operational issues; and, provide other resources identified by the EMCDDA. This report may contain information that might be under verification. The information and resources in this report are intended to strengthen situational awareness within the Network, as well as to help the Network to prepare for, respond to, and recover from public health and social threats caused by new psychoactive substances (NPS) and other substances of interest. A specific focus of this report is to: • highlight recent resources that examine the potential impact of the COVID-19 pandemic on the drug markets and risks to people who use drugs; and, • request that the Network expedite reporting of any event, especially those related to the pandemic, that you consider may have a potential high impact on public health. -

The Next Opioid on the Deadly New Psychoactive
Journal of Analytical Toxicology, 2020;44:937–946 doi:10.1093/jat/bkaa094 Advance Access Publication Date: 3 August 2020 Article Article First Report on Brorphine: The Next Opioid on the Deadly New Psychoactive Substance Downloaded from https://academic.oup.com/jat/article/44/9/937/5879253 by Ghent University user on 07 May 2021 Horizon? Nick Verougstraete1,2,#, Marthe M. Vandeputte1,#, Cathelijne Lyphout3, Annelies Cannaert1, Fabian Hulpia4, Serge Van Calenbergh4, Alain G. Verstraete2,5 and Christophe Stove1,* 1Laboratory of Toxicology, Department of Bioanalysis, Faculty of Pharmaceutical Sciences, Ghent University, Ghent, Belgium; 2Department of Laboratory Medicine, Ghent University Hospital, Ghent, Belgium; 3Emergency Department, Ghent University Hospital, Ghent, Belgium; 4Laboratory for Medicinal Chemistry, Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, Ghent University, Ghent, Belgium and 5Department of Diagnostic Sciences, Ghent University, Ghent, Belgium #These authors contributed equally to this work. *Author to whom correspondence should be addressed. Email: [email protected] Abstract New psychoactive substances continue to appear on the drug market. Until recently, new syn- thetic opioids, which are among the most dangerous new psychoactive substances, primarily encompassed analogs of the potent analgesic fentanyl. Lately, also other new synthetic opioids have increasingly started to surface. This is the first report on the identification and full chemical characterization of brorphine, a novel potent synthetic opioid with a piperidine benzimidazolone structure. A powder, identified as brorphine, was obtained from a patient seeking medical helpfor detoxification. Brorphine was also found in a serum sample of the patient. Liquid chromatography– high-resolution mass spectrometry (LC–HRMS) identified an exact mass of m/z 400.1020 and 402.1005 for the compound, corresponding to both bromine isotopes.