Marine Ecology Progress Series 400:283
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seagrass Meadows As Seascape Nurseries for Rockfish (Sebastes Spp.)
Seagrass meadows as seascape nurseries for rockfish (Sebastes spp.) by Angeleen Olson Bachelor of Science (Honours), Simon Fraser University, 2013 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE in the Faculty of Biology ã Angeleen Olson, 2017 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. ii Supervisory Committee Seagrass meadows as seascape nurseries for rockfish (Sebastes spp.) by Angeleen Olson Bachelor of Science (Honours), Simon Fraser University, 2013 Supervisory Committee Dr. Francis Juanes, Department of Biology Supervisor Dr. Margot Hessing-Lewis, Hakai Institute Co-Supervisor Dr. Rana El-Sabaawi, Department of Biology Departmental Member iii Abstract Nearshore marine habitats provide critical nursery grounds for juvenile fishes, but their functional role requires the consideration of the impacts of spatial connectivity. This thesis examines nursery function in seagrass habitats through a marine landscape (“seascape”) lens, focusing on the spatial interactions between habitats, and their effects on population and trophic dynamics associated with nursery function to rockfish (Sebastes spp.). In the temperate Pacific Ocean, rockfish depend on nearshore habitats after an open-ocean, pelagic larval period. I investigate the role of two important spatial attributes, habitat adjacency and complexity, on rockfish recruitment to seagrass meadows, and the provision of subsidies to rockfish food webs. To test for these effects, underwater visual surveys and collections of young-of-the-year (YOY) Copper Rockfish recruitment (summer 2015) were compared across adjacent seagrass, kelp forest, and sand habitats within a nearshore seascape on the Central Coast of British Columbia. -

Emerging Understanding of Seagrass and Kelp As an Ocean Acidification Management Tool in California
EMERGING UNDERSTANDING OF SEAGRASS AND KELP AS AN OCEAN ACIDIFICATION MANAGEMENT TOOL IN CALIFORNIA Developed by a Working Group of the Ocean Protection Council Science Advisory Team and California Ocean Science Trust AUTHORS Nielsen, K., Stachowicz, J., Carter, H., Boyer, K., Bracken, M., Chan, F., Chavez, F., Hovel, K., Kent, M., Nickols, K., Ruesink, J., Tyburczy, J., and Wheeler, S. JANUARY 2018 Contributors Ocean Protection Council Science Advisory Team Working Group The role of the Ocean Protection Council Science Advisory Team (OPC-SAT) is to provide scientific advice to the California Ocean Protection Council. The work of the OPC-SAT is supported by the California Ocean Protection Council and administered by Ocean Science Trust. OPC-SAT working groups bring together experts from within and outside the OPC-SAT with the ability to access and analyze the best available scientific information on a selected topic. Working Group Members Karina J. Nielsen*, San Francisco State University, co-chair John J. Stachowicz*, University of California, Davis, co-chair Katharyn Boyer, San Francisco State University Matthew Bracken, University of California, Irvine Francis Chan, Oregon State University Francisco Chavez*, Monterey Bay Aquarium Research Institute Kevin Hovel, San Diego State University Kerry Nickols, California State University, Northridge Jennifer Ruesink, University of Washington Joe Tyburczy, California Sea Grant Extension * Denotes OPC-SAT Member California Ocean Science Trust The California Ocean Science Trust (OST) is a non-profit organization whose mission is to advance a constructive role for science in decision-making by promoting collaboration and mutual understanding among scientists, citizens, managers, and policymakers working toward sustained, healthy, and productive coastal and ocean ecosystems. -

DNA Variation and Symbiotic Associations in Phenotypically Diverse Sea Urchin Strongylocentrotus Intermedius
DNA variation and symbiotic associations in phenotypically diverse sea urchin Strongylocentrotus intermedius Evgeniy S. Balakirev*†‡, Vladimir A. Pavlyuchkov§, and Francisco J. Ayala*‡ *Department of Ecology and Evolutionary Biology, University of California, Irvine, CA 92697-2525; †Institute of Marine Biology, Vladivostok 690041, Russia; and §Pacific Research Fisheries Centre (TINRO-Centre), Vladivostok, 690600 Russia Contributed by Francisco J. Ayala, August 20, 2008 (sent for review May 9, 2008) Strongylocentrotus intermedius (A. Agassiz, 1863) is an economically spines of the U form are relatively short; the length, as a rule, does important sea urchin inhabiting the northwest Pacific region of Asia. not exceed one third of the radius of the testa. The spines of the G The northern Primorye (Sea of Japan) populations of S. intermedius form are longer, reaching and frequently exceeding two thirds of the consist of two sympatric morphological forms, ‘‘usual’’ (U) and ‘‘gray’’ testa radius. The testa is significantly thicker in the U form than in (G). The two forms are significantly different in morphology and the G form. The morphological differences between the U and G preferred bathymetric distribution, the G form prevailing in deeper- forms of S. intermedius are stable and easily recognizable (Fig. 1), water settlements. We have analyzed the genetic composition of the and they are systematically reported for the northern Primorye S. intermedius forms using the nucleotide sequences of the mitochon- coast region (V.A.P., unpublished data). drial gene encoding the cytochrome c oxidase subunit I and the Little is known about the population genetics of S. intermedius; nuclear gene encoding bindin to evaluate the possibility of cryptic the available data are limited to allozyme polymorphisms (4–6). -

Coastal Environment and Seaweed-Bed Ecology in Japan
Kuroshio Science 2-1, 15-20, 2008 Coastal Environment and Seaweed-bed Ecology in Japan Kazuo Okuda* Graduate School of Kuroshio Science, Kochi University (2-5-1, Akebono, Kochi 780-8520, Japan) Abstract Seaweed beds are communities consisting of large benthic plants and distributed widely along Japanese coasts. Species constituting seaweed beds in Japan vary depending on localities because of the influence of cold and warm currents. Seaweed beds are important producers along coastal eco- systems in the world, but they have been reduced remarkably in Japan. Not only artificial construc- tions in seashores but also the Phenomenon called “isoyake” have led to the deterioration of coastal environments. To figure out what is going on in nature, Japanese Government began the nationwide survey of seaweed beds. Although trials to recover seaweed communities have been also carrying out, they are not always the solution of subjects. We should think of harmonious coexistence between nature and human being so that problems might not happen. beds and coastal environments; and 5) Towards the sus- Introduction tainable coexistence between nature and human beings Through four billion years of evolution, life on 1. Diversity and distribution of seaweed beds in earth has expanded to almost infinite diversity, with each Japan species interacting with others and molding itself to its habitat until a global ecosystem developed. This diver- Japan has wide range of climate zones from cold sity of life forms is commonly referred to as biodiversity. temperate to subtropical, reflecting its wide geographical Biodiversity is not only crucial to ecosystem balance, but extent from north to south, as well as the influence of also brings great benefit to human lives. -

Blue Carbon Sequestration Along California's Coast
Briefing heldDecember 2020 CCST Expert Briefing Series A Carbon Neutral California One For more details Pager about this briefing: Blue Carbon Sequestration along California’s Coast ccst.us/expert-briefings Select Experts The following experts can advise on Blue Carbon pathways: Joanna Nelson, PhD Founder and Principal LandSea Science [email protected] http://landseascience.com/ Expertise: salt marsh ecology, coastal ecosystem conservation and resilience Lisa Schile-Beers, PhD Senior Associate Silvestrum Climate Associates Figure: Carbon cycles in coastal (blue carbon) habitats (The Watershed Company) Research Associate Smithsonian Environmental Background Research Center • Anthropogenic carbon emissions are a • Blue Carbon refers to carbon stored by [email protected] leading cause of climate change. coastal ecosystems including wetlands, salt Office: (415) 378-2903 marshes, seagrass meadows, and kelp forests. Expertise: wetland and marsh ecology, • California has set an ambitious goal of being carbon cycling, and sea level rise carbon neutral by 2045. • Restoring coastal habitats can increase blue • A combined approach of reducing emissions carbon sequestration and contribute to Melissa Ward, PhD and sequestering carbon – physically state goals. Post-doctoral Researcher removing CO2 from the atmosphere and San Diego State University [email protected] storing it long-term – can help California • Restored coastal habitats also provide many https://melissa-ward.weebly.com reach its goals. other co-benefits. Expertise: carbon storage in seagrass, SEQUESTERING Blue Carbon marsh, and kelp ecosystems in California’s Coastal Ecosystems Lisamarie Per unit area, coastal wetlands, marshes, and Benefits of Blue Carbon Habitats Windham-Myers, PhD eelgrass meadows capture more carbon than 1. Reduced atmospheric CO2 levels Research Ecologist terrestrial habitats such as forests. -

Plymouth Sound and Estuaries SAC: Kelp Forest Condition Assessment 2012
Plymouth Sound and Estuaries SAC: Kelp Forest Condition Assessment 2012. Final report Report Number: ER12-184 Performing Company: Sponsor: Natural England Ecospan Environmental Ltd Framework Agreement No. 22643/04 52 Oreston Road Ecospan Project No: 12-218 Plymouth Devon PL9 7JH Tel: 01752 402238 Email: [email protected] www.ecospan.co.uk Ecospan Environmental Ltd. is registered in England No. 5831900 ISO 9001 Plymouth Sound and Estuaries SAC: Kelp Forest Condition Assessment 2012. Author(s): M D R Field Approved By: M J Hutchings Date of Approval: December 2012 Circulation 1. Gavin Black Natural England 2. Angela gall Natural England 2. Mike Field Ecospan Environmental Ltd ER12-184 Page 1 of 46 Plymouth Sound and Estuaries SAC: Kelp Forest Condition Assessment 2012. Contents 1 EXECUTIVE SUMMARY ..................................................................................................... 3 2 INTRODUCTION ................................................................................................................ 4 3 OBJECTIVES ...................................................................................................................... 5 4 SAMPLING STRATEGY ...................................................................................................... 6 5 METHODS ......................................................................................................................... 8 5.1 Overview ......................................................................................................................... -

CHAPERONE Kelp Vs
4th GRADE Things to do GALLERY Reef Level 1 Level Level 2 Level CHANGING EXHIBIT Tropical Tunnel Tropical GALLERY F I E L D T R I P Tropical CHANGING EXHIBIT Tropical Tunnel Tropical Main Entrance Live Coral Live Tropical Tunnel Tropical TROPICAL PACIFIC GALLERY PACIFIC TROPICAL Main Entrance TROPICAL PACIFIC GALLERY PACIFIC TROPICAL Tropical Preview Tropical CHAPERONE …at the Aquarium Chaperones: Use this guide to move your group through the Gift Store Gift Store • Touch a shark Aquarium’s galleries. The background information, GUIDEguided questions, and activities will keep your • See a show students engaged and actively learning. Sea Otters Honda Theater Sea Otter Honda Theater Sea Otter • Visit a Discovery Lab • Ask questions Surge Channel Surge • Have fun! Kelp NORTHERN PACIFIC GALLERY PACIFIC NORTHERN NORTHERN PACIFIC GALLERY PACIFIC NORTHERN Amber Forest Pool Blue Cavern Blue Cavern Cafe Ray Scuba Blue Ray Pool Cavern Cafe Scuba Ray Pool SOUTHERN CALIFORNIA/BAJA GALLERY SOUTHERN CALIFORNIA/BAJA SOUTHERN CALIFORNIA/BAJA GALLERY SOUTHERN CALIFORNIA/BAJA Seals & Sea Lions Seals & Sea Lions Seals & Sea Lions …back at school Seals & Sea Lions • Write or draw about your trip to the Aquarium Ecosystems • Consider a classroom animal adoption Kelp vs. Coral • Visit aquariumofpacific.org/teachers Welcome to the Kelp Forest and Coral Reef! Shark Lagoon • Keep learning more These unique ecosystems each have producers, Forest like plants and kelp, which take energy from the Lorikeet Shark sun to make their own food, and consumers, Lagoon Shark Lagoon Forest which are incredible animals that rely on producers Lorikeet Blue Cavern, Amber Forest, Kelp Camouflage, Camouflage, Kelp Amber Forest, Blue Cavern, Channel, Sea Surge Otters Ray Pool, and other consumers for food. -

Kelp Forest Seasons of the Sea 89 ©2001 the Regents of the University of California
KELP FOREST SEASONS OF THE SEA FOR THE TEACHER Discipline Earth Science Themes Patterns of Change Key Concept Like land habitats, a kelp forest goes through seasonal changes that effect the animals and plants within the community. Synopsis Students work in groups to act out the seasonal changes and yearly variations that effect the life within a kelp forest. Science Process Skills communicating, comparing, organizing, relating, inferring Social Skills cooperating, sharing and attentive listening Vocabulary benthic, canopy, kelp wrack, herbivore, holdfast, photic zone, phytoplankton, stipe, upwelling, zooplankton MATERIALS Into the Activities Visuals depicting kelp forests: • videos- ("Forests of the Sea", "Ocean Realm", and NOVA'S "Kelp Forest" are some good ones) • slides/or pictures- (check out from MARE library or purchase from Monterey Bay Aquarium) Kelp Forest Seasons of the Sea 89 ©2001 The Regents of the University of California • books- (see literature list, check out from MARE library or purchase from the Monterey Bay Aquarium) butcher, poster or flip chart paper or sentence strips Through the Activities For creating costumes or props: • scissors, glue, staplers • colored construction, tissue or other paper • misc. materials-as needed Beyond the Activities • scenes #1-6 (remove numbers) from each act/copied onto index cards. 1 set for each group of 5-6 students • string or masking tape • butcher, poster or flip chart paper • colored markers or paint INTRODUCTION We often think of marine habitats as being unaffected by the seasonal changes that we experience on land. While seasonal variations in ocean temperature are not as extreme as those experienced on land (e.g., a hot summer day may not effect sea temperature at all), there are seasonal changes within marine ecosystems that have great impact on the plants and animals within them. -

Biodiversity of Kelp Forests and Coralline Algae Habitats in Southwestern Greenland
diversity Article Biodiversity of Kelp Forests and Coralline Algae Habitats in Southwestern Greenland Kathryn M. Schoenrock 1,2,* , Johanne Vad 3,4, Arley Muth 5, Danni M. Pearce 6, Brice R. Rea 7, J. Edward Schofield 7 and Nicholas A. Kamenos 1 1 School of Geographical and Earth Sciences, University of Glasgow, Gregory Building, Lilybank Gardens, Glasgow G12 8QQ, UK; [email protected] 2 Botany and Plant Science, National University of Ireland Galway, Ryan Institute, University Rd., H91 TK33 Galway, Ireland 3 School of Engineering, Geosciences, Infrastructure and Society, Heriot-Watt University, Riccarton Campus, Edinburgh EH14 4AS, UK; [email protected] 4 School of Geosciences, Grant Institute, University of Edinburgh, Edinburgh EH28 8, UK 5 Marine Science Institute, The University of Texas at Austin, College of Natural Sciences, 750 Channel View Drive, Port Aransas, TX 78373-5015, USA; [email protected] 6 Department of Biological and Environmental Sciences, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, Hertfordshire AL10 9AB, UK; [email protected] 7 Geography & Environment, School of Geosciences, University of Aberdeen, Elphinstone Road, Aberdeen AB24 3UF, UK; [email protected] (B.R.R.); j.e.schofi[email protected] (J.E.S.) * Correspondence: [email protected]; Tel.: +353-87-637-2869 Received: 22 August 2018; Accepted: 22 October 2018; Published: 25 October 2018 Abstract: All marine communities in Greenland are experiencing rapid environmental change, and to understand the effects on those structured by seaweeds, baseline records are vital. The kelp and coralline algae habitats along Greenland’s coastlines are rarely studied, and we fill this knowledge gap for the area around Nuuk, west Greenland. -
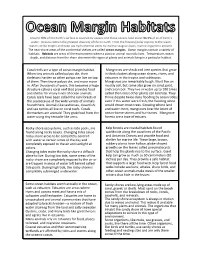
Mangroves Are Shrub and Tree Species That Grow in Thick Clusters Along
Around 70% of the Earth’s surface is covered by oceans and those oceans hold about 96.5% of all of Earth’s water. Oceans contain the greatest diversity of life on Earth. From the freezing polar regions to the warm waters of the tropics and deep sea hydrothermal vents to shallow seagrass beds, marine organisms abound. The near-shore areas of the continental shelves are called ocean margins. Ocean margins contain a variety of habitats. Habitats are areas of the environment where a plant or animal normally lives. Temperature, ocean depth, and distance from the shore determine the types of plants and animals living in a particular habitat. Coral reefs are a type of ocean margin habitat. Mangroves are shrub and tree species that grow When tiny animals called polyps die, their in thick clusters along ocean shores, rivers, and skeletons harden so other polyps can live on top estuaries in the tropics and subtropics. of them. Then those polyps die, and more move Mangroves are remarkably tough. Most live on in. After thousands of years, this becomes a huge muddy soil, but some also grow on sand, peat, structure called a coral reef that provides food and coral rock. They live in water up to 100 times and shelter for many kinds of ocean animals. saltier than most other plants can tolerate. They Corals reefs have been called the rain forests of thrive despite twice-daily flooding by ocean tides; the sea because of the wide variety of animals even if this water were fresh, the flooding alone found there. -

Spatial Vision in the Purple Sea Urchin Strongylocentrotus Purpuratus (Echinoidea)
249 The Journal of Experimental Biology 213, 249-255 Published by The Company of Biologists 2010 doi:10.1242/jeb.033159 Spatial vision in the purple sea urchin Strongylocentrotus purpuratus (Echinoidea) D. Yerramilli and S. Johnsen* Biology Department, Duke University, Durham, NC 27708, USA *Author for correspondence ([email protected]) Accepted 14 October 2009 SUMMARY Recent evidence that echinoids of the genus Echinometra have moderate visual acuity that appears to be mediated by their spines screening off-axis light suggests that the urchin Strongylocentrotus purpuratus, with its higher spine density, may have even more acute spatial vision. We analyzed the movements of 39 specimens of S. purpuratus after they were placed in the center of a featureless tank containing a round, black target that had an angular diameter of 6.5deg. or 10deg. (solid angles of 0.01sr and 0.024sr, respectively). An average orientation vector for each urchin was determined by testing the animal four times, with the target placed successively at bearings of 0deg., 90deg., 180deg. and 270deg. (relative to magnetic east). The urchins showed no significant unimodal or axial orientation relative to any non-target feature of the environment or relative to the changing position of the 6.5deg. target. However, the urchins were strongly axially oriented relative to the changing position of the 10deg. target (mean axis from –1 to 179deg.; 95% confidence interval ± 12deg.; P<0.001, Moore’s non-parametric Hotelling’s test), with 10 of the 20 urchins tested against that target choosing an average bearing within 10deg. of either the target center or its opposite direction (two would be expected by chance). -

Multiple Factors Explain the Covering Behaviour in the Green Sea Urchin, Strongylocentrotus Droebachiensis
ARTICLE IN PRESS ANIMAL BEHAVIOUR, 2007, --, --e-- doi:10.1016/j.anbehav.2006.11.008 Multiple factors explain the covering behaviour in the green sea urchin, Strongylocentrotus droebachiensis CLE´ MENT P. DUMONT*†,DAVIDDROLET*, ISABELLE DESCHEˆ NES* &JOHNH.HIMMELMAN* *De´partement de Biologie, Que´bec-Oce´an, Universite´ Laval yCEAZA, Departamento de Biologia Marina, Universidad Catolica del Norte (Received 26 March 2006; initial acceptance 29 August 2006; final acceptance 13 November 2006; published online ---; MS. number: A10403) Although numerous species of sea urchins often cover themselves with small rocks, shells and algal fragments, the function of this covering behaviour is poorly understood. Diving observations showed that the degree to which the sea urchin Strongylocentrotus droebachiensis covers itself in the field decreases with size. We performed laboratory experiments to examine how the sea urchin’s covering behaviour is affected by the presence of predators, sea urchin size, wave surge, contact with moving algae blades and sunlight. The presence of two common sea urchin predators did not influence the degree to which sea ur- chins covered themselves. Covering responses of sea urchins that were exposed to a strong wave surge and sweeping algal blades were significantly greater than those of individuals that were maintained under still water conditions. The degree to which sea urchins covered themselves in the laboratory also tended to decrease with increasing size. Juveniles showed stronger covering responses than adults, possibly because they are more vulnerable to dislodgement and predation. We found that UV light stimulated a covering response, whereas UV-filtered sunlight and darkness did not, although the response to UV light was much weaker than that to waves and algal movement.