Partial Regulatory Impact Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food and Beverage Industry Insight September 2017
www.peakstone.com Food and Beverage Industry Insight September 2017 0 Food and Beverage Industry Insight | September 2017 Food and Beverage M&A Update Food and Beverage categories continue to be active in M&A activity For 2017 YTD, total U.S. F&B announced transactions of 273 deals are on track to exceed 2016 record levels of 380 Transaction Date Status Target Buyer Size Select Recent Notable Publicly Announced U.S. M&A Transactions Transaction Sep-2017Date Status Target Undisclosed Buyer Size Closed Transaction Date Status Target Buyer Sep-2017 UndisclosedSize Closed Dec-2016Sep-2017 Undisclosed Closed Mar-2016Dec-2016Sep-2017 Undisclosed Closed Mar-2016Dec-2016Jul-2017 Announced $4.20Undisclosed billion ClosedClosed Mar-2016Jun-2017Jul-2017 Announced $14.62$4.20Undisclosed billion billion Closed Jun-2017Jul-2017 Announced $14.62$4.20 billion billion Jun-2017 Announced $14.62 billion www.peakstone.com 1 Food and Beverage Industry Insight | September 2017 Labriola sold to J&J Snack Foods Peakstone served as exclusive financial advisor to Labriola Baking Company, in connection with its sale to J&J Snack Foods, Corp., a leader and innovator in the snack food industry with a $2.4 billion public market capitalization. The transaction is Peakstone's fifth closed sale in 2017 to a multi-billion public company and represents our ability to access large strategic buyers on behalf of our clients. Labriola Baking Company is a premium baker of breads and artisan soft pretzels, located in Alsip, IL. Labriola Baking began in 1993 as a local delivery bakery in the Chicago suburbs and grew into a nationwide provider of superior prebaked breads, rolls, and soft pretzels for retail instore bakery and foodservice outlets. -

Modified UK National Implementation Measures for Phase III of the EU Emissions Trading System
Modified UK National Implementation Measures for Phase III of the EU Emissions Trading System As submitted to the European Commission in April 2012 following the first stage of their scrutiny process This document has been issued by the Department of Energy and Climate Change, together with the Devolved Administrations for Northern Ireland, Scotland and Wales. April 2012 UK’s National Implementation Measures submission – April 2012 Modified UK National Implementation Measures for Phase III of the EU Emissions Trading System As submitted to the European Commission in April 2012 following the first stage of their scrutiny process On 12 December 2011, the UK submitted to the European Commission the UK’s National Implementation Measures (NIMs), containing the preliminary levels of free allocation of allowances to installations under Phase III of the EU Emissions Trading System (2013-2020), in accordance with Article 11 of the revised ETS Directive (2009/29/EC). In response to queries raised by the European Commission during the first stage of their assessment of the UK’s NIMs, the UK has made a small number of modifications to its NIMs. This includes the introduction of preliminary levels of free allocation for four additional installations and amendments to the preliminary free allocation levels of seven installations that were included in the original NIMs submission. The operators of the installations affected have been informed directly of these changes. The allocations are not final at this stage as the Commission’s NIMs scrutiny process is ongoing. Only when all installation-level allocations for an EU Member State have been approved will that Member State’s NIMs and the preliminary levels of allocation be accepted. -

Aldi, West Ewell Date of Visit: 28.07.18
Store and location: Aldi, West Ewell Date of visit: 28.07.18 Brand Product Sugar reduction category Calorie reduction category soft drinks levy Entrance No promotions in entrance Gondola Ends Store layout does not include gondola ends Trolley checkout area The Foodie market Quinoa bars (Coco & cashew) Biscuits n/a The Foodie market Quinoa bars (Goki & cranberry) Biscuits n/a Passions Popcorn (sweet) Sweet Confectionary n/a Passions Popcorn (sweet & salted) Sweet Confectionary n/a Wrigleys Extra chewing gum (peppermint) n/a n/a Wrigleys Extra chewing gum (spearmint) n/a n/a Wrigleys Extra chewing gum (cool breeze) n/a n/a Wrigleys Extra chewing gum (extra white) n/a n/a Passion Deli Pea snacks (sea salt & vingar) n/a Crisps and savoury snacks Passion Deli Pea snacks (sweet chilli) n/a Crisps and savoury snacks The Foodie market Hike protein bars (Cacao) Biscuits n/a The Foodie market Hike protein bars (Berry) Biscuits n/a Dominion Complimints (strongmint) - sugar free n/a n/a Dominion Complimints (spearmint) - sugar free n/a n/a Dominion Complimints (strongmint) - sugar free n/a n/a Passions Deli Red Lentil Snacks (Tangy tomoto) n/a Crisps and savoury snacks Passions Deli Red Lentil Snacks (barbecue) n/a Crisps and savoury snacks Foodie Market Flatbread thin bites (multi-seed) n/a Savoury biscuits, crackers and crispbreads Foodie Market Flatbread thin bites (cheddar & cracked black pepper) n/a Savoury biscuits, crackers and crispbreads Foodie Market Flatbread thin bites (sweet chilli) n/a Savoury biscuits, crackers and crispbreads Dominion -

The Comprehensive Review of Mergers and Acquisitions in the EMEA Region FY 2017 an Acuris Company
An Acuris Company The comprehensive review of mergers and acquisitions in the EMEA region FY 2017 Deal Drivers EMEA mergermarket.com MERGER OR ACQUISITION If you’re serious about achieving the best deal for your business, then you need to get serious about your document preparedness for due diligence. Scrambling for files when there’s an M&A opportunity opens the door for errors, which could harm your shareholders. Businesses rely on Merrill’s award-winning SaaS technology to help them fully prepare for a rapid deal while ensuring their most sensitive documents remain private and secure. To learn how we can help you, contact us today. +44 (0)20 3031 6300 [email protected] www.merrillcorp.com © Merrill Communications LLC. All rights reserved. All trademarks are property of their respective owners. Mergermarket Deal Drivers EMEA Contents 3 FY 2017 Contents Foreword 4 Telecoms, Media & Technology 42 EMEA Heat Chart 5 Transportation 48 All Sectors 6 Pharma, Medical & Biotech 54 Financial Services 18 Construction 60 Industrials & Chemicals 24 The Middle East & North Africa 66 Energy, Mining & Utilities 30 About Merrill Corporation 73 Consumer 36 Merrill Corporation Contacts 73 mergermarket.com Mergermarket Deal Drivers EMEA Foreword 4 FY 2017 Foreword Welcome to the full-year 2017 edition of While dealmaking activity spanned a variety of With strong economic fundamentals, Deal Drivers EMEA, published by Mergermarket sectors, it was industrials & chemicals which heightened boardroom confidence and strong in association with Merrill Corporation. This took the M&A crown in 2017. Driven by large balance sheets underscoring European M&A, report provides an extensive review of M&A deals in the chemical segment and renewed the positive momentum seen in 2017 looks set activity across Europe, the Middle East and interest in the automotive space, deal value to continue into the coming year. -

2019 Annual Report
SOME OF THE PARTS Post Holdings, Inc. 2019 Annual Report NET SALES ADJUSTED EBITDA(1) OPERATING CASH FLOW (in millions) (in millions) (in millions) 718.6 688.0 6,257.2 1,230.7 1,210.4 5,681.1 989.1 5,225.8 933.9 5,026.8 502.4 4,648.2 457.7 386.7 657.4 2015 2016 2017 2018 2019 2015 2016 2017 2018 2019 2015 2016 2017 2018 2019 NET SALES BY CATEGORY 15% Active Nutrition Post Consumer Brands 33% 16% Refrigerated Retail 29% Foodservice Weetabix 7% FINANCIAL HIGHLIGHTS 2015 2016 2017 2018 2019 (in millions except per share data) Net Sales $ 4,648.2 $ 5,026.8 $ 5,225.8 $ 6,257.2 $ 5,681.1 Gross Profit 1,180.0 1,550.5 1,570.8 1,854.0 1,792.1 Operating Profit 218.3 548.8 516.7 573.5 781.0 Net (Loss) Earnings Available to Common Shareholders (132.3) (28.4) 34.8 457.3 121.7 Diluted (Loss) Earnings per Common Share $ (2.33) $ (0.41) $ 0.50 $ 6.16 $ 1.66 Operating Cash Flow 457.7 502.4 386.7 718.6 688.0 Adjusted EBITDA(1) 657.4 933.9 989.1 1,230.7 1,210.4 Adjusted Net Earnings Available to Common Shareholders(1) 35.7 205.8 211.0 318.9 368.8 Adjusted Diluted Earnings per Common Share(1) $ 0.62 $ 2.59 $ 2.67 $ 4.20 $ 4.91 ACTIVELY POSITIONED FOR FUTURE GROWTH BellRing Brands Well positioned as a leader in convenient nutrition, Post’s historical Active Nutrition business, now named BellRing Brands, completed its initial public offering in October 2019 and now trades under the ticker symbol “BRBR” on the New York Stock Exchange. -

The Comprehensive Review of Mergers and Acquisitions in the EMEA Region
An Acuris Company The comprehensive review of mergers and acquisitions in the EMEA region 2017 Half-year edition Deal Drivers EMEA Mergermarket.com Kick back this summer, 1,000,000,000+ let Merrill secure the deal. PAGES UPLOADED 46,000+ VIRTUAL DATA ROOM MERRILL DATASITE PROJECTS SECURED SINCE 2003 Our award-winning Virtual Data Room is used by thousands of leading organisations, worldwide. These organisations trust our team of highly qualified experts to handle their most confidential transactions. 40,000+ Simple. Safe. Secure. Merrill’s technology solutions will take M&A TRANSACTIONS the stress out of your next deal. SINCE 2003 To learn how we can help you, contact us now: +44 20 7562 3200 [email protected] www.merrillcorp.com 22 TECHNOLOGY AWARDS WON IN THE LAST DECADE © Merrill Communications LLC. All rights reserved. All trademarks are property of their respective owners. FINANCIAL TRANSACTIONS & REPORTING | MARKETING & COMMUNICATIONS FOR REGULATED INDUSTRY | CUSTOMER CONTENT & COLLABORATION SOLUTIONS Mergermarket Deal Drivers EMEA Contents 3 Contents Foreword 04 Telecoms, Media & Technology 41 EMEA Heat Chart 05 Transportation 47 All Sectors 06 Pharma, Medical & Biotech 53 Financial Services 17 Construction 59 Industrials & Chemicals 23 The Middle East & North Africa 65 Energy, Mining & Utilities 29 About Merrill Corporation 72 Consumer 35 Merrill Corporation Contacts 72 Mergermarket.com Mergermarket Deal Drivers EMEA Foreword 4 Foreword Welcome to the half-year 2017 edition of negotiations and an uncertain economic Despite ongoing political volatility, it is clear Deal Drivers EMEA, published by Mergermarket future, the UK and Ireland still managed that Europe has retained its status as a fertile in association with Merrill Corporation. -

Company's Reportable
Post Holdings, Inc. 2020 Annual Report Thank You Net Sales This year revealed (in millions) the strength of the 800 construction of our 600 portfolio, and our 400 6,257.2 5,681.1 5,698.7 5,225.8 results give us further 5,026.8 confidence moving 200 forward. However, none 0 of this would have been 2016 2017 2018 2019 2020 possible without the Adjusted EBITDA(1) courage and dedication (in millions) of our people. 1,500 1,200 900 1,230.7 1,210.4 1,140.5 989.1 600 933.9 300 0 2016 2017 2018 2019 2020 Operating Cash Flow (in millions) 800 600 718.6 688.0 625.6 400 502.4 200 386.7 0 2016 2017 2018 2019 2020 Net Sales by Segment 17% BellRing Brands 34% Post Consumer Brands 17% Refrigerated Retail 8% Weetabix 24% Foodservice Financial Highlights (in millions except per share data) 2016 2017 2018 2019 2020 Net Sales $ 5,026.8 $ 5,225.8 $ 6,257.2 $ 5,681.1 $ 5,698.7 Gross Profit 1,550.5 1,570.8 1,854.0 1,792.1 1,787.4 Operating Profit 548.8 516.7 573.5 781.0 700.5 Net (Loss) Earnings Available to Common Shareholders (28.4) 34.8 457.3 121.7 0.8 Diluted (Loss) Earnings per Common Share $ (0.41) $ 0.50 $ 6.16 $ 1.66 $ 0.01 Operating Cash Flow 502.4 386.7 718.6 688.0 625.6 Adjusted EBITDA(1) 933.9 989.1 1,230.7 1,210.4 1,140.5 Adjusted Net Earnings Available to Common Shareholders(1) 205.8 211.0 318.9 378.0 189.8 Adjusted Diluted Earnings per Common Share(1) $ 2.59 $ 2.67 $ 4.20 $ 5.03 $ 2.71 Post Holdings, Inc. -

Post Holdings Annual Report 2019
Post Holdings Annual Report 2019 Form 10-K (NYSE:POST) Published: November 22nd, 2019 PDF generated by stocklight.com UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 _______________________ FORM 10-K _______________________ (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended September 30, 2019 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number: 1-35305 ______________________ POST HOLDINGS, INC. (Exact name of registrant as specified in its charter) _______________________ Missouri 45-3355106 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) 2503 S. Hanley Road St. Louis Missouri 63144 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (314) 644-7600 _______________________ Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $0.01 par value POST New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None _______________________ Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☒ Yes ☐ No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
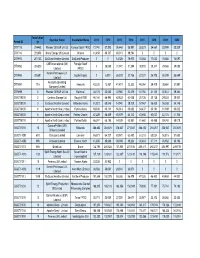
Permit ID Installation ID Operator Name Installation Name 2013 2014
Installation Operator Name Installation Name 2013 2014 2015 2016 2017 2018 2019 2020 Permit ID ID DTI1015 204463 Premier Oil E&P UK Ltd Voyager Spirit FPSO 72,740 37,302 36,642 35,981 35,321 34,660 33,999 33,339 DTI1016 202656 Ithaca Energy UK Limited Athena 41,458 40,737 40,015 39,294 0000 DTI9910 207103 EnQuest Heather Limited EnQuest Producer 0 0 14,726 79,970 78,502 77,033 75,565 74,097 CNR International (UK) Petrojarl Banff DTI9950 206523 0 26,039 31,970 31,394 30,818 30,241 29,665 29,088 Limited FPSO Nexen Petroleum U.K. DTI9960 206591 Golden Eagle 0 6,010 26,378 27,736 27,227 26,718 26,209 25,699 Limited Anasuria Operating DTI9991 715 Anasuria 43,325 13,457 41,811 22,330 40,264 39,479 38,684 37,887 Company Limited DTI9999 8 Premier Oil E&P UK Ltd Balmoral 34,174 33,580 32,980 32,379 31,760 31,140 30,513 29,884 GB-DTI0200 2 Centrica Storage Ltd Rough 47/3B 66,144 64,995 63,833 62,658 30,736 30,136 29,530 28,921 GB-DTI0300 3 EnQuest Heather Limited Kittiwake Alpha 61,921 60,846 14,940 29,329 57,547 56,425 55,288 54,148 GB-DTI0400 4 Apache North Sea Limited Forties Alpha 69,336 68,131 66,913 65,682 64,437 63,181 61,909 60,632 GB-DTI0600 6 Apache North Sea Limited Forties Charlie 47,229 46,409 45,579 44,740 43,892 43,037 42,170 41,300 GB-DTI0700 7 Apache North Sea Limited Forties Delta 66,347 65,195 64,029 62,851 61,660 60,458 59,240 58,019 ConocoPhillips (UK) GB-DTI1000 10 Britannia 286,653 281,674 276,637 271,547 266,402 261,207 255,947 250,670 Britannia Limited GB-DTI-1001 693 Chrysaor Limited Lomond 65,871 64,727 63,570 62,400 61,218 60,024 58,815 57,603 GB-DTI-1002 696 Chrysaor Limited Everest North 41,385 40,666 39,939 39,204 38,461 37,711 36,952 36,190 GB-DTI1003 686 Britoil Ltd Bruce 142,795 280,628 137,805 270,538 265,413 260,237 254,997 249,739 Spirit Energy North Sea Oil Sevan Marine GB-DTI-1010 1123 127,129 124,921 122,687 120,430 118,148 115,844 113,512 111,171 Limited Hummingbird GB-DTI1011 18 Perenco UK Limited Thames Alpha 23,338 22,933 0 0 0000 Nexen Petroleum U.K. -

Applications and Decisions for the East of England
OFFICE OF THE TRAFFIC COMMISSIONER (EAST OF ENGLAND) APPLICATIONS AND DECISIONS PUBLICATION NUMBER: 5208 PUBLICATION DATE: 06/02/2019 OBJECTION DEADLINE DATE: 27/02/2019 Correspondence should be addressed to: Office of the Traffic Commissioner (East of England) Hillcrest House 386 Harehills Lane Leeds LS9 6NF Telephone: 0300 123 9000 Fax: 0113 248 8521 Website: www.gov.uk/traffic-commissioners The public counter at the above office is open from 9.30am to 4pm Monday to Friday The next edition of Applications and Decisions will be published on: 13/02/2019 Publication Price 60 pence (post free) This publication can be viewed by visiting our website at the above address. It is also available, free of charge, via e-mail. To use this service please send an e-mail with your details to: [email protected] APPLICATIONS AND DECISIONS Important Information All correspondence relating to public inquiries should be sent to: Office of the Traffic Commissioner (East of England) Eastbrook Shaftesbury Road Cambridge CB2 8DR The public counter in Cambridge is open for the receipt of documents between 9.30am and 4pm Monday to Friday. There is no facility to make payments of any sort at the counter. General Notes Layout and presentation – Entries in each section (other than in section 5) are listed in alphabetical order. Each entry is prefaced by a reference number, which should be quoted in all correspondence or enquiries. Further notes precede each section, where appropriate. Accuracy of publication – Details published of applications reflect information provided by applicants. The Traffic Commissioner cannot be held responsible for applications that contain incorrect information. -

2017 Annual Report Net Sales by Category Weetabix
NEW HORIZONS Post Holdings, Inc. 2017 Annual Report Net Sales by Category Weetabix Active Nutrition 2% Post Consumer Brands 14% 35% Private Brands 8% pasta 5% branded potatoes and cheese 9% value-added egg products 27% Michael Foods Group Net Sales Adjusted EBITDA(1) Operating Cash Flow ($ in millions) ($ in millions) ($ in millions) $6,000 $1,200 $600 $4,000 $800 $400 502.4 989.1 5,225.8 5,026.8 933.9 451.6 4,648.2 386.7 $2,000 $400 $200 657.4 216.7 1,034.1 84.1 2,411.1 344.5 $0 $0 $0 163.0 2013 2014 2015 2016 2017 2013 2014 2015 2016 2017 2013 2014 2015 2016 2017 Financial Highlights (in millions except per share data) 2013 2014 2015 2016 2017 Net Sales $ 1,034.1 $ 2,411.1 $ 4,648.2 $ 5,026.8 $ 5,225.8 Gross Profit 424.9 621.2 1,174.4 1,547.4 1,574.1 Operating Profit (Loss) 107.8 (207.7) 212.7 545.7 520.3 Net Earnings (Loss) Available to Common Stockholders 9.8 (358.6) (132.3) (28.4) 34.8 Diluted Net Earnings (Loss) per Common Share $ 0.30 $ (9.03) $ (2.33) $ (0.41) $ 0.50 Operating Cash Flow 84.1 163.0 451.6 502.4 386.7 Adjusted EBITDA(1) 216.7 344.5 657.4 933.9 989.1 Adjusted Net Earnings (Loss) Available to Common Stockholders(1) 31.1 (16.6) 35.7 205.8 211.0 Adjusted Diluted Net Earnings (Loss) per Common Share(1) $ 0.94 $ (0.42) $ 0.62 $ 2.59 $ 2.67 Post Holdings, Inc. -

Regulations 2005
Consultation draft 29.3.07 STATUTORY INSTRUMENTS 2007 No. FOOD, ENGLAND The Food for Particular Nutritional Uses (Miscellaneous Amendments) (England) Regulations 2007 Made - - - - 2007 Laid before Parliament 2007 Coming into force - - 2007 The Secretary of State makes the following Regulations in exercise of the powers conferred by section 2(2) of the European Communities Act 1972(a). The Secretary of State has been designated for the purpose of that section in relation to measures relating to food (including drink) including the primary production of food(b). As required by Article 9 of Regulation (EC) No. 178/2002 of the European Parliament and of the Council laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety(c), there has been open and transparent public consultation during the preparation and evaluation of these Regulations. Title, application and commencement 1. These Regulations may be cited as the Food for Particular Nutritional Uses (Miscellaneous Amendments) (England) Regulations 2007; they apply in relation to England only and come into force on 2007. Amendment of the Infant Formula and Follow-on Formula Regulations 1995 2.—(1) The Infant Formula and Follow-on Formula Regulations 1995(d) are amended in accordance with paragraph (2). (2) In regulation 22 (offences and enforcement)— (a) in paragraph (1), insert at the beginning the words “Subject to paragraph (3),”; and (b) after paragraph (2A), add the paragraph— “(3) A person shall not be considered to have contravened or failed to comply with the provisions contained in regulations 2 or 5 where the sale or export which would otherwise (a) 1972 c.68.