Not a Ureilite, Maybe a Brachinite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Olivine-Dominated Asteroids and Meteorites: Distinguishing Nebular and Igneous Histories
Meteoritics & Planetary Science 42, Nr 2, 155–170 (2007) Abstract available online at http://meteoritics.org Olivine-dominated asteroids and meteorites: Distinguishing nebular and igneous histories Jessica M. SUNSHINE1*, Schelte J. BUS2, Catherine M. CORRIGAN3, Timothy J. MCCOY4, and Thomas H. BURBINE5 1Department of Astronomy, University of Maryland, College Park, Maryland 20742, USA 2University of Hawai‘i, Institute for Astronomy, Hilo, Hawai‘i 96720, USA 3Johns Hopkins University, Applied Physics Laboratory, Laurel, Maryland 20723–6099, USA 4Department of Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560–0119, USA 5Department of Astronomy, Mount Holyoke College, South Hadley, Massachusetts 01075, USA *Corresponding author. E-mail: [email protected] (Received 14 February 2006; revision accepted 19 November 2006) Abstract–Melting models indicate that the composition and abundance of olivine systematically co-vary and are therefore excellent petrologic indicators. However, heliocentric distance, and thus surface temperature, has a significant effect on the spectra of olivine-rich asteroids. We show that composition and temperature complexly interact spectrally, and must be simultaneously taken into account in order to infer olivine composition accurately. We find that most (7/9) of the olivine- dominated asteroids are magnesian and thus likely sampled mantles differentiated from ordinary chondrite sources (e.g., pallasites). However, two other olivine-rich asteroids (289 Nenetta and 246 Asporina) are found to be more ferroan. Melting models show that partial melting cannot produce olivine-rich residues that are more ferroan than the chondrite precursor from which they formed. Thus, even moderately ferroan olivine must have non-ordinary chondrite origins, and therefore likely originate from oxidized R chondrites or melts thereof, which reflect variations in nebular composition within the asteroid belt. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Brachinite-Paper.Pdf
Open Research Online The Open University’s repository of research publications and other research outputs Petrological, petrofabric, and oxygen isotopic study of five ungrouped meteorites related to brachinites Journal Item How to cite: Hasegawa, Hikari; Mikouchi, Takashi; Yamaguchi, Akira; Yasutake, Masahiro; Greenwood, Richard and Franchi, Ian A. (2019). Petrological, petrofabric, and oxygen isotopic study of five ungrouped meteorites related to brachinites. Meteoritics & Planetary Science, 54(4) pp. 752–767. For guidance on citations see FAQs. c 2019 The Meteoritical Society https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Proof Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.1111/maps.13249 Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk M A P S 13249-3018 Dispatch: 30.1.19 CE: Malarvizhi Journal Code Manuscript No. No. of pages: 16 PE: Nishanthan P. Meteoritics & Planetary Science 1–16 (2019) 1 doi: 10.1111/maps.13249 2 3 4 5 Petrological, petrofabric, and oxygen isotopic study of five ungrouped meteorites 6 related to brachinites 7 8 9 Hikari HASEGAWA 1*, Takashi MIKOUCHI1,2, Akira YAMAGUCHI 3,4, 10 1 Masahiro YASUTAKE5, Richard C. GREENWOOD6, and Ian A. FRANCHI6 11 12 1Department of Earth and Planetary Science, Graduate School of Science, University of Tokyo, Hongo, Bunkyo-ku, Tokyo 13 113-0033, -

Meteorite Collections: Sample List
Meteorite Collections: Sample List Institute of Meteoritics Department of Earth and Planetary Sciences University of New Mexico October 01, 2021 Institute of Meteoritics Meteorite Collection The IOM meteorite collection includes samples from approximately 600 different meteorites, representative of most meteorite types. The last printed copy of the collection's Catalog was published in 1990. We will no longer publish a printed catalog, but instead have produced this web-based Online Catalog, which presents the current catalog in searchable and downloadable forms. The database will be updated periodically. The date on the front page of this version of the catalog is the date that it was downloaded from the worldwide web. The catalog website is: Although we have made every effort to avoid inaccuracies, the database may still contain errors. Please contact the collection's Curator, Dr. Rhian Jones, ([email protected]) if you have any questions or comments. Cover photos: Top left: Thin section photomicrograph of the martian shergottite, Zagami (crossed nicols). Brightly colored crystals are pyroxene; black material is maskelynite (a form of plagioclase feldspar that has been rendered amorphous by high shock pressures). Photo is 1.5 mm across. (Photo by R. Jones.) Top right: The Pasamonte, New Mexico, eucrite (basalt). This individual stone is covered with shiny black fusion crust that formed as the stone fell through the earth's atmosphere. Photo is 8 cm across. (Photo by K. Nicols.) Bottom left: The Dora, New Mexico, pallasite. Orange crystals of olivine are set in a matrix of iron, nickel metal. Photo is 10 cm across. (Photo by K. -
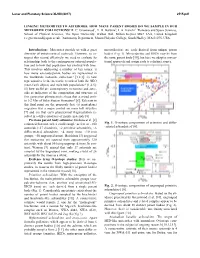
Linking Meteorites to Asteroids: How Many Parent Bodies Do We Sample in Our Meteorite Collections? R
Lunar and Planetary Science XLVIII (2017) 2515.pdf LINKING METEORITES TO ASTEROIDS: HOW MANY PARENT BODIES DO WE SAMPLE IN OUR METEORITE COLLECTIONS? R. C. Greenwood1, T. H. Burbine2, I. A. Franchi1 1Planetary and Space Sciences, School of Physical Sciences, The Open University, Walton Hall, Milton Keynes MK7 6AA, United Kingdom [email protected], 2Astronomy Department, Mount Holyoke College, South Hadley, MA 01075, USA. Introduction: Meteorites provide us with a great mesosiderites) are each derived from unique parent diversity of extraterrestrial materials. However, to in- bodies (Fig. 1). Mesosiderites and HEDs may be from terpret this record effectively we need to evaluate its the same parent body [10], but here we adopt a conven- relationship, both to the contemporary asteroid popula- tional approach and assign each to a distinct source. tion and to how that population has evolved with time. This involves addressing a number of key issues: i) how many asteroids/parent bodies are represented in the worldwide meteorite collection? [1,2,3]; ii) how representative is the meteorite record of both the NEO (near-Earth object) and main belt populations? [1,4,5]; iii) how useful are contemporary meteorites and aster- oids as indicators of the composition and structure of first generation planetesimals; those that accreted with- in 1-2 Myr of Solar System formation? [6]. Relevant to this final point are the proposals that: (i) giant planet migration was a major control on main belt structure [7] and (ii) that early planetesimal fragmentation re- sulted in a differential loss of mantle materials [8]. Previous parent body estimates: Burbine et al. -

UNIVERSITY of CALIFORNIA, SAN DIEGO Primitive and Differentiated
UNIVERSITY OF CALIFORNIA, SAN DIEGO Primitive and differentiated achondrite meteorites and partial melting in the early Solar System A Thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Earth Sciences By Christopher Andrew Corder Committee in charge: Professor James M. D. Day, Chair Professor Geoffrey W. Cook Professor David R. Stegman 2015 © Christopher Andrew Corder, 2015 All rights reserved. The Thesis of Christopher Corder is approved and it is acceptable in quality and form for publication on microfilm and electronically: Chair University of California, San Diego 2015 iii Dedication This manuscript would be far from complete without thanking those who helped set the stage for the many hours of work it documents, and those thank-yous would be far from complete without recognizing my parents and their unwavering support throughout years of study. I know I was the one in the lab, but I never would have made it there, or to my defense, or through any of the long days and nights if it weren’t for you both. Thank you, so much. I’d love to thank my entire family, especially Stephen. You’re a brother Stephen, and you encourage me more than you know. Whenever it seemed like research was going nowhere, you were there to remind me why science is always a worthwhile endeavor. Even more importantly you remind me, by example, that there are exceptional people in this world. Many thanks are due to the lab group I worked with and other smart folks at SIO. First of all, thank you James for trusting this intriguing suite of meteorites to my care. -

Trace Element Chemistry of Cumulus Ridge 04071 Pallasite with Implications for Main Group Pallasites
Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites Item Type Article; text Authors Danielson, L. R.; Righter, K.; Humayun, M. Citation Danielson, L. R., Righter, K., & Humayun, M. (2009). Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites. Meteoritics & Planetary Science, 44(7), 1019-1032. DOI 10.1111/j.1945-5100.2009.tb00785.x Publisher The Meteoritical Society Journal Meteoritics & Planetary Science Rights Copyright © The Meteoritical Society Download date 23/09/2021 14:17:54 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final published version Link to Item http://hdl.handle.net/10150/656592 Meteoritics & Planetary Science 44, Nr 7, 1019–1032 (2009) Abstract available online at http://meteoritics.org Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites Lisa R. DANIELSON1*, Kevin RIGHTER2, and Munir HUMAYUN3 1Mailcode JE23, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 2Mailcode KT, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 3National High Magnetic Field Laboratory and Department of Geological Sciences, Florida State University, Tallahassee, Florida 32310, USA *Corresponding author. E-mail: [email protected] (Received 06 November 2008; revision accepted 11 May 2009) Abstract–Pallasites have long been thought to represent samples from the metallic core–silicate mantle boundary of a small asteroid-sized body, with as many as ten different parent bodies recognized recently. This report focuses on the description, classification, and petrogenetic history of pallasite Cumulus Ridge (CMS) 04071 using electron microscopy and laser ablation ICP-MS. -

53Mn-53Cr SYSTEMATICS of the BRACHINITE NWA 4882. D. R. Dunlap1, S
79th Annual Meeting of the Meteoritical Society (2016) 6217.pdf 53Mn-53Cr SYSTEMATICS OF THE BRACHINITE NWA 4882. D. R. Dunlap1, S. J. Romaniello1, and M. Wadhwa1, 1Center for Meteorite Studies, School of Earth and Space Exploration, Arizona State University Tempe, AZ 85287 ([email protected]). Introduction: High-resolution timescales of planetary accretion and differentiation can be interrogated through 53 53 the application of the Mn- Cr relative chronometer. Manganese-53 has a short half-life (t1/2 = 3.7 Ma) and decays to 53Cr making it ideally suited to resolve time differences between objects forming within the first ~20 Ma of Solar Sytem history. The 53Mn-53Cr chronometer has been successfully applied by many studies in recent years (e.g., [1- 5]). Additionally, the mass independent anomalies in 54Cr can serve as a powerful genetic tracer of meteorite parent bodies (e.g., [6-8] and references therein). In this study, we have made high precision analyses of the Mn-Cr isotope systematics in the brachinite North- west Africa (NWA) 4882 as part of our broader investigations of the high resolution chronologies of primitive and ungrouped achondrites. The NWA 4882 brachinite is a coarse-grained dunite with a protogranular texture composed dominantly of olivine (Fa35) with minor clinopyroxene (Fs9.3 Wo47.1), K-poor plagioclase (An35.-37.2 Or0.3-0.5), chro- mite, iron sulfide, and kamacite [9]. Methods: All sample handling, mineral separation and ion exchange chromatography procedures were per- formed under clean laboratory conditions in the Isotope Cosmochemistry and Geochronology Lab (ICGL) at Arizo- na State University (ASU). -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

Kleines Lehrbuch Der Astronomie Und Astrophysik Band 9
Kleines Lehrbuch der Astronomie und Astrophysik M. Scholz Band 9: Meteore und Meteorite Meteoroide, Meteorite und Meteorströme, Interplanetare Materie Kleines Lehrbuch der Astronomie und Astrophysik Band 9 M. Scholz Kleines Lehrbuch der Astronomie und Astrophysik Band 9: Meteore und Meteorite Meteoroide, Meteorite und Meteorströme, Interplanetare Materie E-Book-Ausgabe 2009 Das Werk einschließlich aller seiner Teile ist urheberrechtlich geschützt. Jede Verwertung außerhalb der engen Grenzen des Urheberrechtsgesetzes ist ohne Zustimmung des Autors unzulässig. Bildnachweis: Wikipedia Commons, NASA, ESA, Autor M.Scholz Kleines Lehrbuch der Astronomie und Astrophysik Band 9 Meteore und Meteorite Meteoroide, Meteorite und Meteorströme, Interplanetare Materie Ausgabe 2009 [email protected] INHALTSVERZEICHNIS METEOROIDE, METEORITE UND METEORSTRÖME .......................................................................... 2 METEORE ......................................................................................................................................................... 3 Meteorbeobachtung ........................................................................................................................................................ 5 Meteorströme ............................................................................................................................................................. 10 Feuerkugeln und Meteoritenfälle ............................................................................................................................... -

Meteorite Collections: Catalog
Meteorite Collections: Catalog Institute of Meteoritics Department of Earth and Planetary Sciences University of New Mexico July 25, 2011 Institute of Meteoritics Meteorite Collection The IOM meteorite collection includes samples from approximately 600 different meteorites, representative of most meteorite types. The last printed copy of the collection's Catalog was published in 1990. We will no longer publish a printed catalog, but instead have produced this web-based Online Catalog, which presents the current catalog in searchable and downloadable forms. The database will be updated periodically. The date on the front page of this version of the catalog is the date that it was downloaded from the worldwide web. The catalog website is: Although we have made every effort to avoid inaccuracies, the database may still contain errors. Please contact the collection's Curator, Dr. Rhian Jones, ([email protected]) if you have any questions or comments. Cover photos: Top left: Thin section photomicrograph of the martian shergottite, Zagami (crossed nicols). Brightly colored crystals are pyroxene; black material is maskelynite (a form of plagioclase feldspar that has been rendered amorphous by high shock pressures). Photo is 1.5 mm across. (Photo by R. Jones.) Top right: The Pasamonte, New Mexico, eucrite (basalt). This individual stone is covered with shiny black fusion crust that formed as the stone fell through the earth's atmosphere. Photo is 8 cm across. (Photo by K. Nicols.) Bottom left: The Dora, New Mexico, pallasite. Orange crystals of olivine are set in a matrix of iron, nickel metal. Photo is 10 cm across. (Photo by K. -

The Meteoritical Bulletin, O. 95
Meteoritics & Planetary Science 44, Nr 3, 1–33 (2009) Abstract available online at http://meteoritics.org The Meteoritical Bulletin, o. 95 Michael K. WEISBERG1, 2*, Caroline SMITH3, 4, Gretchen BENEDIX3, Luigi FOLCO5, Kevin RIGHTER6, Jutta ZIPFEL7, Akira YAMAGUCHI8, and Hasnaa CHENNAOUI AOUDJEHANE9 1Department of Physical Science, Kingsborough Community College and the Graduate School of the City University of New York, 2001 Oriental Blvd., Brooklyn, New York 11235, USA 2Department of Earth and Planetary Science, American Museum of Natural History, Central Park West New York, New York 10024, USA 3Department of Mineralogy, The Natural History Museum, Cromwell Road, London SW7 5BD, UK 4School of Geographical and Earth Science, University of Glasgow, Scotland, G12 8QQ, UK 5Museo Nazionale dell’Antartide, Università di Siena, Via Laterina 8, I-53100 Siena, Italy 6Mailcode KT, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 7Sektion Meteoritenforschung, Forschungsinstitut und Naturmuseum Senckenberg, Senckenberganlage 25, D-60325, Frankfurt am Main, Germany 8Antarctic Meteorite Research Center, National Institute of Polar Research, 1-9-10 Kaga, Itabashi, Tokyo 173-8515, Japan 9Université Hassan II Casablanca, Faculté des sciences, Département de Géologie, BP 5366, Mâarif, Casablanca, Morocco *Corresponding author. E-mail: [email protected] (Received 25 February 2009) Abstract–The Meteoritical Bulletin No. 95 reports 1093 (282 non-Antarctic and 801 Antarctic) newly approved meteorite names and their recovery histories, macroscopic descriptions, petrography, mineral compositions and geochemistry. Meteorites reported include lunar meteorites, eucrites, mesosiderites, angrites, ureilites, an acapulcoite, and H, L, LL, R, CO, CM, CK and CV chondrites. Three new falls, the Bunburra Rockhole (Australia) eucrite and the recent (Nov., 2008) Buzzard Coulee (Canada) H4 chondrite, and Tamdakht (Morocco) H5 chondrite are reported.