Evolution of the Patellar Sesamoid Bone in Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of the Patellar Sesamoid Bone in Mammals
A peer-reviewed version of this preprint was published in PeerJ on 21 March 2017. View the peer-reviewed version (peerj.com/articles/3103), which is the preferred citable publication unless you specifically need to cite this preprint. Samuels ME, Regnault S, Hutchinson JR. 2017. Evolution of the patellar sesamoid bone in mammals. PeerJ 5:e3103 https://doi.org/10.7717/peerj.3103 Evolution of the patellar sesamoid bone in mammals Mark E Samuels 1, 2 , Sophie Regnault 3 , John R Hutchinson Corresp. 3 1 Department of Medicine, University of Montreal, Montreal, Quebec, Canada 2 Centre de Recherche du CHU Ste-Justine, Montreal, Quebec, Canada 3 Structure & Motion Laboratory, Department of Comparative Biomedical Sciences, The Royal Veterinary College, Hatfield, Hertfordshire, United Kingdom Corresponding Author: John R Hutchinson Email address: [email protected] The patella is a sesamoid bone located in the major extensor tendon of the knee joint, in the hindlimb of many tetrapods. Although numerous aspects of knee morphology are ancient and conserved among most tetrapods, the evolutionary occurrence of an ossified patella is highly variable. Among extant (crown clade) groups it is found in most birds, most lizards, the monotreme mammals and almost all placental mammals, but it is absent in most marsupial mammals as well as many reptiles. Here we integrate data from the literature and first-hand studies of fossil and recent skeletal remains to reconstruct the evolution of the mammalian patella. We infer that bony patellae most likely evolved between four to six times in crown group Mammalia: in monotremes, in the extinct multituberculates, in one or more stem-mammal genera outside of therian or eutherian mammals, and up to three times in therian mammals. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Abstracts Volume
SVPCA 58, Cambridge 2010, Preface Welcome to the 58th Symposium of Vertebrate Palaeontology and Comparative Anatomy, hosted by the University Museum of Zoology, Cambridge. We very much hope you find the meeting rewarding, and that you have time to enjoy the other attractions of the university, colleges, and city centre. The meeting logo was designed by Julia Molnar1 and depicts the finback whale (Balaenoptera physalus) on display above the Museum of Zoology. Enjoy your time in Cambridge! 1JuliaMolnar,RoyalVeterinaryCollege,jmolnar-at-rvc.ac.uk 1 SPPC/SVPCA 2010 Programme Overview Tuesday 14th September: SPPC Session 1, Chair: Matt Lowe 15.30-15.50 Christian Baars Infacol – if it’s good enough for babies, it’s good enough for ammonites. 15.50-15.10 Nigel R Larkin The joy of steel: How to master your awkward fossil in the field. 16.10-16.30 Frank Osbaeck Mechanical and chemical preparation methods used on the lower Eocene cementstone concretions from the Mo-Clay of northern Denmark 19.00 onwards EVENING RECEPTION, ZOOLOGY MUSEUM Wednesday 15th September 9.10-9.20 Welcome, notices for the day Session 1, Chair: Paul Barret 9.20-9.40 Eric Buffetaut et al. The Early Cretaceous vertebrate assemblage from the Napai Formation of Guangxi, southern China: a comparison with Thai assemblages 9.40-10.00 Michael J Benton et al. Recovery and expansion of marine vertebrate faunas in the Triassic of South China 10.00-10.20 Susan E Evans and Magdalend Borsuk-Bialynicka The Early Triassic faunal assemblage of Czatkowice, Poland 10.20-10.40 Sweetman, Steven C The first records of amphibians, lizards and maniraptoran dinosaurs from the Lower Cretaceous Hastings Group of south-east England 10.40-11.10 COFFEE BREAK Session 2, Chair: Jenny Clack 11.10-11.30 Tim Smithson and Stan Wood Eliminating Romer’s Gap: new tetrapods from the basal Carboniferous of the Scottish Borders 2 11.30-11.50 Per E Ahlberg et al. -

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
_____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ I MUNIS ENTOMOLOGY & ZOOLOGY Ankara / Turkey II _____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ Scope: Munis Entomology & Zoology publishes a wide variety of papers on all aspects of Entomology and Zoology from all of the world, including mainly studies on systematics, taxonomy, nomenclature, fauna, biogeography, biodiversity, ecology, morphology, behavior, conservation, paleobiology and other aspects are appropriate topics for papers submitted to Munis Entomology & Zoology. Submission of Manuscripts: Works published or under consideration elsewhere (including on the internet) will not be accepted. At first submission, one double spaced hard copy (text and tables) with figures (may not be original) must be sent to the Editors, Dr. Hüseyin Özdikmen for publication in MEZ. All manuscripts should be submitted as Word file or PDF file in an e-mail attachment. If electronic submission is not possible due to limitations of electronic space at the sending or receiving ends, unavailability of e-mail, etc., we will accept “hard” versions, in triplicate, accompanied by an electronic version stored in a floppy disk, a CD-ROM. Review Process: When submitting manuscripts, all authors provides the name, of at least three qualified experts (they also provide their address, subject fields and e-mails). Then, the editors send to experts to review the papers. The review process should normally be completed within 45-60 days. After reviewing papers by reviwers: Rejected papers are discarded. For accepted papers, authors are asked to modify their papers according to suggestions of the reviewers and editors. Final versions of manuscripts and figures are needed in a digital format. -

The Cambridge Dictionary of Human Biology and Evolution Larry L
Cambridge University Press 0521664861 - The Cambridge Dictionary of Human Biology and Evolution Larry L. Mai, Marcus Young Owl and M. Patricia Kersting Excerpt More information Abdur Reef A. n dates: see Oakley’s dating series in box below. A antigen: epitope that specifies the A in the ABO AAA: abbreviation for several societies of interest to blood group. It consists of four precursor sugars human evolutionary biologists, including the attached to glycoproteins of the cell membrane, American Anthropological Association and the aka H substance, plus a specific fifth terminal sugar, American Anatomical Association. N-acetylgalactosamine, that is attached by an enzyme. A Oakley’s absolute dating series A.1 date: highest of Oakley’s hierarchical levels of absolute dating, the direct dating of a specimen, e.g. by measuring the radiocarbon activity of a bone itself. A.2 date: one of Oakley’s hierarchical levels of absolute dating, dating derived from direct determination by physical measurement of the age of the sediments containing the fossil specimen. A.3 date: one of Oakley’s hierarchical levels of absolute dating, the correlation of a fossil-bearing horizon with another deposit whose age has been determined directly by A.1 or A.2 methods. See biostratigraphy. A.4 date: lowest of Oakley’s hierarchical levels of absolute dating, estimating an absolute age on the basis of some theoretical consideration, such as matching climatic fluctuations observed in strata with astro- nomically derived curves of effective solar radiation, or matching terrestrial glacial and interglacial episodes with the known marine paleotemperature or oxygen isotope stage. (Cf. -

8. Primate Evolution
8. Primate Evolution Jonathan M. G. Perry, Ph.D., The Johns Hopkins University School of Medicine Stephanie L. Canington, B.A., The Johns Hopkins University School of Medicine Learning Objectives • Understand the major trends in primate evolution from the origin of primates to the origin of our own species • Learn about primate adaptations and how they characterize major primate groups • Discuss the kinds of evidence that anthropologists use to find out how extinct primates are related to each other and to living primates • Recognize how the changing geography and climate of Earth have influenced where and when primates have thrived or gone extinct The first fifty million years of primate evolution was a series of adaptive radiations leading to the diversification of the earliest lemurs, monkeys, and apes. The primate story begins in the canopy and understory of conifer-dominated forests, with our small, furtive ancestors subsisting at night, beneath the notice of day-active dinosaurs. From the archaic plesiadapiforms (archaic primates) to the earliest groups of true primates (euprimates), the origin of our own order is characterized by the struggle for new food sources and microhabitats in the arboreal setting. Climate change forced major extinctions as the northern continents became increasingly dry, cold, and seasonal and as tropical rainforests gave way to deciduous forests, woodlands, and eventually grasslands. Lemurs, lorises, and tarsiers—once diverse groups containing many species—became rare, except for lemurs in Madagascar where there were no anthropoid competitors and perhaps few predators. Meanwhile, anthropoids (monkeys and apes) emerged in the Old World, then dispersed across parts of the northern hemisphere, Africa, and ultimately South America. -

Lower Triassic Postcanine Teeth with Allotherian-Like Crowns
Research Letters South African Journal of Science 103, May/June 2007 245 Lower Triassic postcanine teeth with allotherian-like crowns F. Abdala*‡, H. Mocke*§ and P.J. Hancox* The Allotheria are fossil mammals with upper and lower post- canines usually showing two longitudinal rows of cusps separated by a central valley. The group comprises the poorly known haramiyids, mostly represented by isolated teeth, and the notably diverse and long-lived multituberculates; its monophyly is uncer- tain. The oldest records of this particular group are the Late Triassic (Norian–Rhaetian) haramiyids. We present here postcanines with haramiyid-like crowns that were recovered from the Lower Triassic of South Africa. A distinguishing feature of the new teeth is that they are single-rooted. This is the oldest record of mammal-like teeth with crowns having parallel rows of cusps, representing a temporal extension of some 43 million years from similar crown patterns of haramiyids and tritylodontids. This finding reinforces evidence of the remarkable faunal turnover of therapsids in the Early/Middle Triassic, at which time an explosive origin followed by a rapid early diversification of herbivorous/omnivorous forms with occluding expanded postcanines took place. Introduction The Beaufort Group of the South African Karoo shows an abundance and diversity of non-mammalian synapsids, which have allowed for biostratigraphic subdivisions ranging from Middle Permian to Middle Triassic.1 The youngest of these Fig. 1.Allotherian-like teeth.A, Occlusal and lateral views of BP/1/6515 (Pattern 1); B, occlusal and lateral views of BP/1/6516 (Pattern 2). biozones, the Cynognathus Assemblage Zone (AZ), comprises the full extent of the Burgersdorp Formation of the Tarkastad Sub- found to be most parsimonious from an unconstrained search, group (J. -
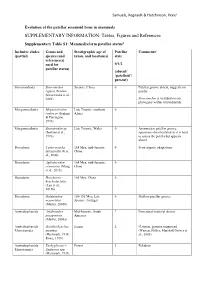
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Eutheria (Placental Mammals)
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction have supernumerary teeth (e.g. some whales, armadillos, Eutheria (or Placentalia) is the most taxonomically diverse etc.), in extant placentals the number of teeth is at most of three branches or clades of mammals, the other two three upper and lower incisors, one upper and lower being Metatheria (or Marsupialia) and Prototheria (or canine, four upper and lower premolars, and three upper Monotremata). When named by Gill in 1872, Eutheria and lower molars. Except for one fewer upper molar, a included both marsupials and placentals. It was Huxley in domestic dog retains this pattern. Compared to reptiles, 1880 that recognized Eutheria basically as used today to mammals have fewer skull bones through fusion and loss, include only placentals. McKenna and Bell in their although bones are variously emphasized in each of the Classification of Mammals, published in 1997, chose to three major mammalian taxa. use Placentalia rather than Eutheria to avoid the confusion Physiologically, mammals are all endotherms of varying of what taxa should be included in Eutheria. Others such as degrees of efficiency. They are also homeothermic with a Rougier have used Eutheria and Placentalia in the sense relatively high resting temperature. These characteristics used here. Placentalia includes all extant placentals and are also found in birds, but because of anatomical their most recent common ancestor. -

Carnegie Institution of Washington Monograph Series
BTILL UMI Carnegie Institution of Washington Monograph Series BT ILL UMI 1 The Carnegie Institution of Washington, D. C. 1902. Octavo, 16 pp. 2 The Carnegie Institution of Washington, D. C. Articles of Incorporation, Deed of Trust, etc. 1902. Octavo, 15 pp. 3 The Carnegie Institution of Washington, D. C. Proceedings of the Board of Trustees, January, 1902. 1902. Octavo, 15 pp. 4 CONARD, HENRY S. The Waterlilies: A Monograph of the Genus Nymphaea. 1905. Quarto, [1] + xiii + 279 pp., 30 pls., 82 figs. 5 BURNHAM, S. W. A General Catalogue of Double Stars within 121° of the North Pole. 1906. Quarto. Part I. The Catalogue. pp. [2] + lv + 1–256r. Part II. Notes to the Catalogue. pp. viii + 257–1086. 6 COVILLE, FREDERICK VERNON, and DANIEL TREMBLY MACDOUGAL. Desert Botani- cal Laboratory of the Carnegie Institution. 1903. Octavo, vi + 58 pp., 29 pls., 4 figs. 7 RICHARDS, THEODORE WILLIAM, and WILFRED NEWSOME STULL. New Method for Determining Compressibility. 1903. Octavo, 45 pp., 5 figs. 8 FARLOW, WILLIAM G. Bibliographical Index of North American Fungi. Vol. 1, Part 1. Abrothallus to Badhamia. 1905. Octavo, xxxv + 312 pp. 9 HILL, GEORGE WILLIAM, The Collected Mathematical Works of. Quarto. Vol. I. With introduction by H. POINCARÉ. 1905. xix + 363 pp. +errata, frontispiece. Vol. II. 1906. vii + 339 pp. + errata. Vol. III. 1906. iv + 577 pp. Vol. IV. 1907. vi + 460 pp. 10 NEWCOMB, SIMON. On the Position of the Galactic and Other Principal Planes toward Which the Stars Tend to Crowd. (Contributions to Stellar Statistics, First Paper.) 1904. Quarto, ii + 32 pp.