Enpro Journal Volume 4, No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Web Magazine
Printable Web Mag Version - InterfaithFamily.com Page 1 of 3 Home > > Printable Web Mag Version HOME RESOURCE PAGES Web Magazine Hindu-Jewish Relationships CURRENT WEB MAGAZINE ISSUE Issue 188: July 25, 2006 FEATURED ARTICLES ARTICLE ARCHIVE Uniting of the Tribes: Our Hindu- CONNECTIONS IN YOUR AREA Jewish Wedding By Jason Jay and Alaka Ray JOIN THE DISCUSSION Who knew that a foodfight with yellow turmeric paste could turn in-laws into a family? IFF NETWORK Read More ABOUT IFF Your Daughter Has Something to Tell You... PRESS ROOM By Jana Sikdar When an Indian girl dates a Jewish girl, it's STORE a test of tolerance for two modern families. Read More Seeds Search: By Reika Dutta Her Hindu family celebrated when her sister married a Jewish man, but suspicious stereotypes are creeping into their conversations. Read More ALSO IN THIS ISSUE Featured Partners/Funders/Links More Articles on Hindu-Jewish Relationships Walking Seven Circles By Tony Castleman A Jewish man and his Hindu wife face the question of how to raise the children and decide two religions are better than Login one. Coming to Terms with My Son's Choices Login Name: http://www.interfaithfamily.com/site/c.ekLSK5MLIrG/b.1711661/k.7AD9/Printable_Web... 7/27/2006 Printable Web Mag Version - InterfaithFamily.com Page 2 of 3 By Sallie Teitelbaum Castleman Password: Tony Castleman's mother struggles with her son's decision to do both. Not Signed Up? Find Out More. My Jewish Son's Engagement to a Hindu Woman: Pluses and Minuses By Rifka Klein The cultural differences are strange, but the love and support are familiar. -

Traditional Costume Heritage of Bangladesh and How It Is Connected to Our Culture
American Scientific Research Journal for Engineering, Technology, and Sciences (ASRJETS) ISSN (Print) 2313-4410, ISSN (Online) 2313-4402 © Global Society of Scientific Research and Researchers http://asrjetsjournal.org/ Traditional Costume Heritage of Bangladesh and How it is Connected to Our Culture Shakinaz Mahamud* Lecturer, Fashion Design & Technology Department, BGMEA University of Fashion & Technology (BUFT), Dhaka, Bangladesh Email: [email protected] Abstract Bangladesh has mixed culture and there is a diversified influence of culture on our costume. The main purposes of this survey are to find the connection between culture & costumes. Commonly Bangladeshi women like to wear saree, Salwar kameez , men wear Panjabi, Pajama, Fatua, Lungi ,Dhoti, & Gumcha and modern Bangladeshi like to wear formal shirt, pant, polo shirt, T- shirt & suit . Purposes of the research are to represent our traditional costumes and how it is connected to our culture and traditional heritage. Keywords: Culture; Traditional ; Costume; Saree; Lungi ; Dhoti ; Gamcha ; Panjabi. 1. Introduction The Civilization of Bangladesh is a hundred year old. Once upon a time in Bangla as the richest region of the Indian subcontinent, that time many countries, people came to the Bangla business purpose and some people were permanently staying in Bangla and marriage Bangle people. That time Bangle people influenced by different types of cultural day by day they absorb that culture after that, British east India Company start to come in the subcontinent near about seventy centuries 1947 East India company left the subcontinent but many European reaming in Bangla / subcontinent. Costumes represent cultures of a country. Due to mixed culture influence dress up of Bangladeshi has mixed flavors. -

Consumer Product Related to Wedding Ceremony (Retail and Wholesale)
Consumer product related to wedding ceremony (Retail and Wholesale) Consumer product related to wedding ceremony (Retail and Wholesale) A. Industry Classification: The lower medial income country, economy of Bangladesh has been performing well during the last decades. GDP1 growth rate ups and down within 6% to 6.5% during this time. In 2015, per-capita income stood at $1,314. Total GDP in 2015 is $209 billion, 44th in world ranking, and sectoral contribution of GDP in agriculture sector 19%; industry sector 30% and services sector 51%. Although geographically, Bangladesh is small country but demographically it is not because it has more than 160 million populations from which 87.5 million are in the labour force. Occupational distribution of lebour force in agriculture sector is 40%, industry sector 30% and services sector 30% and only 4.5% are unemployed. The huge amount of labour force leads to develop various industries such as textiles, food processing, steel, pulp and paper, jute, shipbuilding, pharmaceuticals, electronics, automotive parts, ceramics, fertiliser, construction materials, leather, natural gas, renewable energy etc. During the last decades, there was several changes such as new employment sectors increased; unemployment decreased; purchasing power increased due to the increase in income level; at the same time consumption also increased proportionally; also luxuries and occasional consumer products demand increased as well. So, the retail and wholesale business enlarge across the country which represents huge amount of domestic trade. About consumer products: By definition, consumer products or goods are any tangible products or final goods for sale that are used by a person or household for non-business purposes or direct consumption. -

SONG CODE and Send to 4000 to Set a Song As Your Welcome Tune
Type WT<space>SONG CODE and send to 4000 to set a song as your Welcome Tune Song Name Song Code Artist/Movie/Album Aaj Apchaa Raate 5631 Anindya N Upal Ami Pathbhola Ek Pathik Esechhi 5633 Hemanta Mukherjee N Asha Bhosle Andhakarer Pare 5634 Somlata Acharyya Chowdhury Ashaa Jaoa 5635 Boney Chakravarty Auld Lang Syne And Purano Sei Diner Katha 5636 Shano Banerji N Subhajit Mukherjee Badrakto 5637 Rupam Islam Bak Bak Bakam Bakam 5638 Priya Bhattacharya N Chorus Bhalobese Diganta Diyechho 5639 Hemanta Mukherjee N Asha Bhosle Bhootader Bechitranusthan 56310 Dola Ganguli Parama Banerjee Shano Banerji N Aneek Dutta Bhooter Bhobishyot 56312 Rupankar Bagchi Bhooter Bhobishyot karaoke Track 56311 Instrumental Brishti 56313 Anjan Dutt N Somlata Acharyya Chowdhury Bum Bum Chika Bum 56315 Shamik Sinha n sumit Samaddar Bum Bum Chika Bum karaoke Track 56314 Instrumental Chalo Jai 56316 Somlata Acharyya Chowdhury Chena Chena 56317 Zubeen Garg N Anindita Chena Shona Prithibita 56318 Nachiketa Chakraborty Deep Jwele Oi Tara 56319 Asha Bhosle Dekhlam Dekhar Par 56320 Javed Ali N Anwesha Dutta Gupta Ei To Aami Club Kolkata Mix 56321 Rupam Islam Ei To Aami One 56322 Rupam Islam Ei To Aami Three 56323 Rupam Islam Ei To Aami Two 56324 Rupam Islam Ek Jhatkay Baba Ma Raji 56325 Shaan n mahalakshmi Iyer Ekali Biral Niral Shayane 56326 Asha Bhosle Ekla Anek Door 56327 Somlata Acharyya Chowdhury Gaanola 56328 Kabir Suman Hate Tomar Kaita Rekha 56329 Boney Chakravarty Jagorane Jay Bibhabori 56330 Kabir Suman Anjan Dutt N Somlata Acharyya Chowdhury Jatiswar 56361 -

The Glory of Bangladeshi Saree and Its Various Aspects
© 2018 JETIR December 2018, Volume 5, Issue 12 www.jetir.org (ISSN-2349-5162) The Glory of Bangladeshi Saree and its Various Aspects Nadia Tasnim Piya BGMEA University of Fashion & Technology Abstract Bangladesh has diverse traditional aspects and a variety in the cultural influence on our costume. For Bangladesh; Costume is an impactful part which cannot be departed from our core culture as the connection between culture & costumes are always perceptible. While talking about costume; “Saree” is one of the focal points which got huge significance in overall Bangladeshi costume tradition. Being the national costume; Saree is a garment which is still in fashion since ancient time to now being unchanged. The purpose of this research is to signify our traditional costume; “Saree” and the way it’s connected to our own traditional and cultural heritage. Thus in this study; the main intention of author is to light up on various aspects of “Saree” which considered as the glory of Bangladeshi women since history to modern time. Author mainly put her focus on the historical background of Saree, its different parts, various draping styles, Saree in different culture, occasional uses and variety of Saree according to its quality etc. In short; through this research; one can, get the real gems and the over view concerning several different aspects of legendary Bangladeshi Saree. Key words: Bangladeshi Saree, Draping style, Jamdani, Muslin, Rajshai Silk, Tangail Saree. 1 Introduction Bangladesh is famous for its rich and diverse tradition that reflects through culture, clothing, music, art, dance, film, festivals, occasions, architecture, livelihood, literature and language and the behavior of individuals etc. -

Book-6-Hindu-Marriage FINAL
NEW AGE PUROHIT DARPAN! Bd¤¢eL f¤f¤f¤EEEl¡¢qa cfÑZcfÑZcfÑZ Book 6 HINDU MARRIAGE ¢q¾c¥ ¢hh¡q ¡¡¡ Purohit (priests) Kanai L. Mukherjee – Bibhas Bandyopadhyay Editor Aloka Chakravarty Publishers Association of Grandparents of Indian Immigrants, USA POBox 50032, Nashvillle, TN 37205 ([email protected]) Distributor Eagle Book Bindery Cedar Rapids. IA 52405 Fourth Edition NEW AGE PUROHIT DARPAN LIST OF ORIGINAL AUDIO TRACKS Listen to the audio by control+click on the list of audios given here. Here is the link for the list of audios: www.agiivideo.com/books/audio/hindumarriage Audio Pages Titles Links 1 14 Preliminaries http://www.agiivideo.com/books/audio/hindumarriage/Audio-01- Preliminaries-p14.mp3 2 30 Nandimukh http://www.agiivideo.com/books/audio/hindumarriage/Audio-02- Nandimukh-p30.mp3 3 59 Marriage http://www.agiivideo.com/books/audio/hindumarriage/Audio-03- Marriage-p59.mp3 4 84 Marriage http://www.agiivideo.com/books/audio/hindumarriage/Audio-04- conclusion Marriage-conclusion-p84.mp3 ii NEW AGE PUROHIT DARPAN Global Communication Dilip Som Art work Monidipa Basu iii NEW AGE PUROHIT DARPAN o^ mm bREt h©dyQ d}Atu, mm icšmn¤Ú icšQ EthÙ¹u | jj h¡Q−jLje¡ S¥oü hªqØf¢aÙ¹ÅÅÅ¡Å ¢ek¤eš²¥ jqÉw zz6zz Om mama bratey hridayam dadhatu, mama chitta manu chittam tehantu | Mama bachamekamana jushaswa Brihaspatistwa nijunaktu majyam ||6|| Let your heart be with my mission May your mind and my mind be one. May our words delight each other. May Divinity unite the two of us. Our publications Book 1: Saraswati Puja Book 2: Lakshmi Puja Book 3: -
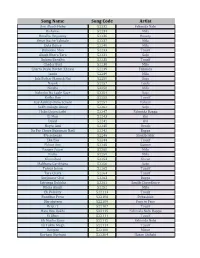
Song Name Song Code Artist
Song Name Song Code Artist Ami Akash Hobo 52232 Fahmida Nabi Bishshas 52234 Mila Bondhu Doyamoy 52236 Beauty Bristi Nache Taletale 52237 Mila Dola Dance 52240 Mila Bishonno Mon 52233 Tausif Akash Bhora Tara 52231 Saju Bolona Bondhu 52235 Tausif Chader Buri 52238 Mila Charta Deyal Hothat Kheyal 52239 Fahmida Jaadu 52249 Mila Jala Bojhar Manush Nai 52250 Saju Nayok 52257 Leela Nirobe 52258 Mila Kolonko Na Lagle Gaye 52254 Saju Kotha Dao 52255 Tausif Kay Aankay Onno Schobi 52251 Tahsan Sokhi Bologo Amay 52262 Saju Hobo Dujon Sathi 52247 Fahmida Bappa Ei Mon 52243 Oni Doyal 52241 Ovi Hoyto Ami 52248 Ornob Du Par Chuya Bohoman Nadi 52242 Bappa Eto sohosay 52246 Shakib Jakir Eka Eka 52244 Tausif Ekhon Ami 52245 Sumon Paaper Pujari 52260 Mila Nisha 52259 Mila Khunshuti 52253 Shuvo Malikana Garikhana 52256 Saju Tomar Jonno 52265 Tausif Tare Chara 52264 Tausif Surjosane Chol 52263 Bappa Satranga Dukkha 52261 Sanjib Chowdhury Khola Akash 52252 Mila Ek Polokey 522113 Tausif Bondhur Prem 522106 Debashish Dhrubotara 522109 Face to Face Bristi 2 522107 Tausif Hate Deo Rakhi 522115 Fahmida Nobi Bappa Ei Jibon 522111 Tausif Ek Mutho Gaan 522112 Fahmida Nobi Ek Tukro Megh 522114 Tausif Danpite 522108 Minar Kurbani Kurbani 522304 Hasan Shihabi Premchara Cholena Duniya 522342 Saju Rodela Dupur 522348 Tahsan Mithila Jeona Durey Chole 522280 Bappa Toni Khachar Bhitor Ochin Pakhi 522291 Labonno Kumari 522303 Shohor Bondi Nach mayuri nach re 522341 Labonno Tumi Amar 522381 Shahed Chandkumari 522385 Purno Soilo Soi 522391 Purno Itihas 71 522534 ROCK 404 Chandrabindu -

A Study of the Impacts of Men's International Labour Migration on Th
Bangor University DOCTOR OF PHILOSOPHY Wives left behind: A study of the impacts of men’s international labour migration on their wives in Bangladesh Kamal, Marzana Award date: 2020 Awarding institution: Bangor University Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 Wives left behind: A study of the impacts of men’s international labour migration on their wives in Bangladesh Marzana Kamal A thesis submitted to the School of History, Philosophy and Social Sciences, for the degree of Doctor of Philosophy in Sociology and Social Policy August 2019 Table of Contents Acknowledgements ................................................................................................................ 11 Declaration............................................................................................................................. -

An Overview of the Westin Dhaka (From Sales & Marketing Perspective)
AN OVERVIEW OF THE WESTIN DHAKA (FROM SALES & MARKETING PERSPECTIVE) Internship Report on AN OVERVIEW OF THE WESTIN DHAKA (FROM SALES & MARKETING PERSPECTIVE) Submitted To: Suntu Kumar Ghosh Assistant Professor BRAC Business School Submitted By: Sumaiya Noor ID: 09304022 BRAC Business School Date of Submission: 7th May, 2013 BRAC UNIVERSITY Letter of Transmittal 7th May, 2013 Suntu Kumar Ghosh Assistant Professor BRAC Business School BRAC University Subject: Submission of Internship report Dear Sir, This is my great pleasure to submit the Internship report “Overview of The Westin Dhaka (From sales & marketing perspective)”.It is prepared depending on my three months long Internship program in The Westin Dhaka at Gulshan-2. This report has been prepared to fulfill the requirement of my internship program at my assigned organization in The Westin Dhaka. I have put my best effort to make this report a successful one. It has been joyful & enlightening experience for me to work in the organization & prepare this report. However this has been obviously a great source of learning for me to conduct similar types of studies in the future. I would like to express my sincere gratitude to you for your kind guidance & suggestions in preparing the report. It would my immense pleasure if you find this report useful & informative to have an apparent perspective on the issue. I shall be happy to provide any further explanation regarding this report if required & please do not hesitate to call me if you have any query on this report or any other relevant matters. Thanks and Best Regards, Sumaiya Noor ID: 09304022 Department: BBS BRAC University ACKNOWLEDGEMENT It is said that “Tow heads are better than one”. -

Bangladesh a Nation on the March Commemorative Publication on the Occasion of 43Rd Anniversary of the Independence and National Day of Bangladesh-2014
0 Bangladesh A Nation on the March Commemorative publication on the occasion of 43rd Anniversary of the Independence and National Day of Bangladesh-2014 Bangladesh High Commission, Canberra 1 12 Chaitra 1420 26 March 2014 Message of the President On the eve of our great Independence and National Day, I extend my heartfelt greetings and felicitations to the fellow countrymen living at home and abroad. The great Independence Day is a glorious one in our national life. We achieved our long-cherished independence through a nine-month long armed struggle and manifold forbearance and patience. Today, I recall with deep gratitude the heroic martyrs who made supreme sacrifices in achieving our independence. On this historic day, I recall with profound respect Father of the Nation Bangabandhu Sheikh Mujibur Rahman who proclaimed country’s independence despite myriad challenges and odds. I also recall with deep reverence our four National Leaders; valiant freedom-fighters, organizers, supporters and people from all walks of life for their unmatched contributions and courageous role that accelerated the achievement of our victory. The contributions of those valiant sons would be written in golden letters in the history of our independence forever. One of the prime objectives of our hard-earned independence was to build a happy and prosperous Bangladesh. Keeping that in mind, the Government has been putting untiring efforts in materializing the objectives of independence. We have been achieved some significant progress in the fields of agriculture, education, health, rural infrastructure, communication, energy and power, trade & commerce, ICT, SME, youth & sports, women and children, women empowerment, etc. -

Syncretism in Religious and Cultural Dimension: an Empirical Study Among Hindu, Muslim and Christian Community in Chapra Block, Nadia, West Bengal
International Journal of Humanities and Social Science Research International Journal of Humanities and Social Science Research ISSN: 2455-2070; Impact Factor: RJIF 5.22 Received: 30-01-2019; Accepted: 02-03-2019 www.socialsciencejournal.in Volume 5; Issue 3; May 2019; Page No. 01-06 Syncretism in religious and cultural dimension: An empirical study among Hindu, Muslim and Christian community in Chapra block, Nadia, West Bengal Rimi Sarkar1*, Samita Manna2 1 ICSSR Doctoral Fellow, University of Kalyani, Nadia, West Bengal, India 2 Professor of Sociology, University of Kalyani, Nadia, West Bengal, India Abstract Syncretism has recently re-emerged as a valuable tool for understanding the complex dynamics of ethnicity, interconnectedness and post-modernism. Religious syncretism is exhibits blending of two or more religious belief systems into a new system, or may be due to the incorporation into a religious tradition of beliefs from unrelated traditions. Cultural syncretism is the spontaneous normal ongoing process of assimilation. With the passage of time different groups or communities adopt many new cultural traits and absorb themselves within it. The study is also trying to reveal the nature of assimilation in various forms like the syncretism in the form of Salad Bowl, syncretism in the form of Sandwich or the simple borrowing cultural traits from the dominating groups. Through this process of assimilation, the people of various communities exchange their beliefs, customs and practises and create a new mixed cultural form to accommodate them in the larger horizon of the society. Keywords: cultural syncretism, religious syncretism, religious diversity, mixed culture Introduction own unity and their differences from other such India is a country known for its unity among diversity. -

THE UNIVERSITY of HULL from Pioneers to New Millennials A
THE UNIVERSITY OF HULL From Pioneers to New Millennials A dynamics of identity among British Bangladeshi women in London Thesis presented for the degree of Doctorate of Philosophy in the University of Hull by Lubna Ferdowsi School of Education and Social Sciences The University of Hull July 2017 Abstract This dissertation is an ethnography about British Bangladeshi women of different age groups who live in London. In this study I explore the identity dynamics of these women. I do this through an intersectional approach, focussing on age, generation, socio- economic status, and time of migration. I argue that the term ‗generation‘, which has been used in existing literature on diaspora and migration, is confusing and inappropriate to address the diversity of diaspora people in relation to their intersectional and contextual differences. Hence, a significant finding is that using ‗cohort‘ can be an appropriate way to avoid generalizing diasporan, and address diversity among them and the different contexts in which they are situated. My participants have been through distinctive experiences in their process of migration, most at different and particular stages of their life cycles, and in some cases, even women in the same age groups have had different contextual or transnational upbringing in the pre and post migration phases. Therefore, arguing that the term ‗generation‘ is confounding, I have preferred to categorise my participants as members of particular ‗cohorts‘ from an ethnographic perspective through intersecting their age, time of migration, and contextual upbringing. I have termed them as follows: the Pioneer Cohort, the Cooked in Britain Cohort, the British-born Cohort and the New-migrant Cohort.