Review Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CASE REPORT Uterine Rupture Due to Invasive Metastatic
UC Irvine Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health Title Uterine Rupture due to Invasive Metastatic Gestational Trophoblastic Neoplasm Permalink https://escholarship.org/uc/item/7064k01v Journal Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health, 14(5) ISSN 1936-900X Authors Bruner, David I Pritchard, Amy M Clarke, Jonathan E Publication Date 2013 DOI 10.5811/westjem.2013.4.15868 License https://creativecommons.org/licenses/by-nc/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California CASE REPORT Uterine Rupture Due to Invasive Metastatic Gestational Trophoblastic Neoplasm David I. Bruner, MD* * Naval Medical Center Portsmouth, Emergency Medicine Program, Portsmouth, Virginia Amy M. Pritchard, DO† † Naval Medical Hospital Camp Pendleton, Oceanside, California Jonathan Clarke, MD‡ ‡ Naval Medical Center Jacksonville, Jacksonville, Florida Supervising Section Editor: Rick McPheetors, DO Submission history: Submitted January 13, 2013; Revision received April 9, 2013; Accepted April 11, 2013 Full text available through open access at http://escholarship.org/uc/uciem_westjem DOI: 10.5811/westjem.2013.4.15868 While complete molar pregnancies are rare, they are wrought with a host of potential complications to include invasive gestational trophoblastic neoplasia. Persistent gestational trophoblastic disease following molar pregnancy is a potentially fatal complication that must be recognized early and treated aggressively for both immediate and long-term recovery. We present the case of a 21-year-old woman with abdominal pain and presyncope 1 month after a molar pregnancy with a subsequent uterine rupture due to invasive gestational trophoblastic neoplasm. We will discuss the complications of molar pregnancies including the risks and management of invasive, metastatic gestational trophoblastic neoplasia. -

New Management of Gestational Trophoblastic Diseases; a Continuum of Moles to Choriocarcinoma: a Review Article
©2018 ASP Ins., Afarand Scholarly Publishing Institute, Iran ISSN: 2476-5848; Journal of Obstetrics, Gynecology and Cancer Research. 2018;3(3):123-128. New Management of Gestational trophoblastic diseases; A Continuum of Moles to Choriocarcinoma: A Review Article A R T I C L E I N F O A B S T R A C T Introduction Article Type Gestational trophoblastic diseases (GTD) is the only group of female reproductive neoplasms derived from paternal genetic material (Androgenic origin). GTD Analytical Review Authors is a continuum from benign to malignant; molar pregnancy is benign, but choriocarcinoma 1 MD is malignant. Approximately 45% of patients have metastatic disease when Gestational 1 MD trophoblastic neoplasia (GTN) is diagnosed. GTN is unique in women malignancies because 2 Soheila Aminimoghaddam* MD, PhD it arises from trophoblast but not from genital organs. It is curable with chemotherapy, Nastaran Abolghasem , Tahereh Ashraf- Ganjooie , low-risk GTN completely response to single-agent chemotherapy and does not require Conclusionhistological confirmation. In persistent GTN, clinical staging and workup of metastasis should be performed. The aim of the present study was to review the new management of GTD. In the case of brain, liver, or renal metastases, any woman of reproductive age How to cite this article who presents with an apparent metastatic malignancy of unknown primary site should be screened for the possibility of GTN with a serum HCG level. Excisional biopsy is not indicated to histologically confirm the diagnosis of malignant GTN if the patient is not pregnant and Soheila Aminimoghaddam, Nas- taran Abolghasem, Tahereh As-hraf- has a high HCG value. -

Rotana Alsaggaf, MS
Neoplasms and Factors Associated with Their Development in Patients Diagnosed with Myotonic Dystrophy Type I Item Type dissertation Authors Alsaggaf, Rotana Publication Date 2018 Abstract Background. Recent epidemiological studies have provided evidence that myotonic dystrophy type I (DM1) patients are at excess risk of cancer, but inconsistencies in reported cancer sites exist. The risk of benign tumors and contributing factors to tu... Keywords Cancer; Tumors; Cataract; Comorbidity; Diabetes Mellitus; Myotonic Dystrophy; Neoplasms; Thyroid Diseases Download date 07/10/2021 07:06:48 Link to Item http://hdl.handle.net/10713/7926 Rotana Alsaggaf, M.S. Pre-doctoral Fellow - Clinical Genetics Branch, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH PhD Candidate – Department of Epidemiology & Public Health, University of Maryland, Baltimore Contact Information Business Address 9609 Medical Center Drive, 6E530 Rockville, MD 20850 Business Phone 240-276-6402 Emails [email protected] [email protected] Education University of Maryland – Baltimore, Baltimore, MD Ongoing Ph.D. Epidemiology Expected graduation: May 2018 2015 M.S. Epidemiology & Preventive Medicine Concentration: Human Genetics 2014 GradCert. Research Ethics Colorado State University, Fort Collins, CO 2009 B.S. Biological Science Minor: Biomedical Sciences 2009 Cert. Biomedical Engineering Interdisciplinary studies program Professional Experience Research Experience 2016 – present Pre-doctoral Fellow National Cancer Institute, National Institutes -

Obstetric Gynecologic Radiology OB001-EB-X MRI Virtual Hysterosalpingography: How to Do It?
Obstetric Gynecologic Radiology OB001-EB-X MRI Virtual Hysterosalpingography: How to do it? All Day Room: OB Community, Learning Center Participants Patricia M. Carrascosa, MD, Buenos Aires, Argentina (Presenter) Research Consultant, General Electric Company Jimena B. Carpio, MD, Buenos Aires, Argentina (Abstract Co-Author) Nothing to Disclose Javier Vallejos, MD, Vicente Lopez, Argentina (Abstract Co-Author) Nothing to Disclose Carlos Capunay, MD, Buenos Aires, Argentina (Abstract Co-Author) Nothing to Disclose TEACHING POINTS To describe a novel radiation free imaging modality for integral the evaluation of the female pelvis.To be familiar with the advantages, disadvantages, normal anatomy, pathological findings and pitfalls of MRI virtual hysterosalpingography. TABLE OF CONTENTS/OUTLINE A. Description of the procedure. Patient preparation B. MRI image acquisition. Technical parameters. C. Contrast injection protocol. D. Image post-processing and Interpretation E. Diagnostic Imaging F. Indications G. Outcomes OB002-EB-X Common and Uncommon Imaging Presentations of Ectopic Pregnancy All Day Room: OB Community, Learning Center Participants Kellan Schallert, MD, Charlottesville, VA (Presenter) Nothing to Disclose Gia A. Deangelis, MD, Charlottesville, VA (Abstract Co-Author) Nothing to Disclose Arun Krishnaraj, MD, MPH, Charlottesville, VA (Abstract Co-Author) Nothing to Disclose TEACHING POINTS Illustrate the imaging features of various locations and appearances of ectopic pregnancies Highlight classic features, features which portend a poor prognosis, and unique locations of ectopic pregnancies Brief review of the management options TABLE OF CONTENTS/OUTLINE Common and Uncommon Imaging Presentations of Ectopic PregnancyTeaching Points Illustrate the imaging features of various locations and appearances of ectopic pregnancy Highlight classic features, features which portend a poor prognosis, and unique locations of ectopic pregnancies Brief review of the management optionsOutline1. -

Gestational Trophoblastic Disease
GESTATIONAL Danielle Krause, MD PGY3 TROPHOBLASTIC Obstetrics & DISEASE Gynecology Gestational Trophoblastic Disease ■ Spectrum of interrelated conditions ■ All arise from the placenta ■ Abnormal trophoblastic proliferation ■ All secrete human chorionic gonadotropin (hCG) – Excellent biomarker for disease progression and treatment response Benign •Partial hydatiform mole •Complete hydatiform mole Gestational Trophoblastic Malignant Disease •Invasive and metastatic mole •Choriocarcinoma •Placental Site Trophoblastic Tumor •Epithelioid Trophoblastic Tumor •New: Atypical placental site nodule Epidemiology ■ Hydatiform mole (Benign) – 1/1000 pregnancies worldwide – High-income populations ■ Complete moles: 1-3/1000 ■ Partial moles: 3/1000 – More common at extremes of reproductive age (<15 and >45yo) – History of previous molar pregnancy 10x risk *Biggest RF ■ Choriocarcinoma – North America/Europe: 1/40,000 – Southeast Asia/Japan: 3-4/40,000 Benign •Partial hydatiform mole •Complete hydatiform mole Gestational Trophoblastic Malignant Disease •Invasive and metastatic mole •Choriocarcinoma •Placental Site Trophoblastic Tumor •Epithelioid Trophoblastic Tumor •New: Atypical placental site nodule Review of Fertilization 1. Sperm head binds to zona pellucida (glycoprotein layer surrounding the oocyte) 2. Acrosome reaction = hydrolytic enzymes released at the head of the sperm 3. Penetration of the zona pellucida 4. Cell membrane of sperm and egg fuse 5. Sperm nucleus/cytoplasm released into the egg 6. Completion of second meiotic division by the -

Intraplacental Choriocarcinoma: a Report of Two Cases
J Clin Pathol: first published as 10.1136/jcp.41.10.1085 on 1 October 1988. Downloaded from J Clin Pathol 1988;41:1085-1088 Intraplacental choriocarcinoma: a report of two cases H FOX,* R N LAURINIt From the *Department ofPathology, University ofManchester, and tDivision ofDevelopmental and Paediatric Pathology, Institut de Pathologie, Centre Hospitalier Universitaire, Vaudois, Lausanne, Switzerland SUMMARY Two examples ofintraplacental choriocarcinoma are described. Both were small and had arisen in otherwise normal third trimester placentas. The covering mantle ofmany ofthe villi adjacent to the choriocarcinomas was formed, either focally or wholly, of neoplastic trophoblastic tissue: it is only at this stage of the development of a choriocarcinoma that villous structures are present, and a study ofthese cases adds further evidence for an origin ofchoriocarcinoma from villous trophoblast. Intraplacental choriocarcinomas can give rise to both maternal and fetal metastases during pregnancy, and it is suggested that such lesions also serve as an origin for those choriocarcinomas which follow a term pregnancy. In western countries about 20% of gestational Histopathologicalfindings choriocarcinomas follow an apparently normal full Sections from the reddish area showed a typical term pregnancy.' It has been generally assumed that in choriocarcinoma (fig 1); in some areas there was a such cases the trophoblastic neoplasm arises either sharp transition from choriocarcinoma to normal villi from villous tissue which has been retained within the -

Pattern of Gynaecological Malignancies in a Tertiary Care Hospital
Open Journal of Obstetrics and Gynecology, 2019, 9, 449-457 http://www.scirp.org/journal/ojog ISSN Online: 2160-8806 ISSN Print: 2160-8792 Pattern of Gynaecological Malignancies in a Tertiary Care Hospital Sayma Afroz*, Gulshan Ara, Fahmida Sultana Deparment of Obstetrics & Gynaecology, Enam Medical College & Hospital, Dhaka, Bangladesh How to cite this paper: Afroz, S., Ara, G. Abstract and Sultana, F. (2019) Pattern of Gynaeco- logical Malignancies in a Tertiary Care Background: Gynaecological malignancies are the second most common Hospital. Open Journal of Obstetrics and cancer of females after cancer breast. Gynaecological malignancies contribute Gynecology, 9, 449-457. significantly to cancer burden and have a higher rate of mortality and mor- https://doi.org/10.4236/ojog.2019.94044 bidity. Carcinoma cervix is the commonest gynaecological malignancy in de- Received: March 8, 2019 veloping countries while in developed countries, ovarian cancer is the com- Accepted: April 8, 2019 monest. Comprehensive statistics on gynecologic malignancies reported from Published: April 11, 2019 Bangladesh are deficient. This study was performed to ascertain the profile of Copyright © 2019 by author(s) and gynecologic cancers reported at our center regarding demography, the Scientific Research Publishing Inc. frequency of involvement at various sites, clinical presentation, incidence, This work is licensed under the Creative histologic subtypes and stage at presentation. Methods: This is a retrospec- Commons Attribution International tive study where the records of the Departments of Gynecology and Patholo- License (CC BY 4.0). http://creativecommons.org/licenses/by/4.0/ gy at Enam Medical College & Hospital, Dhaka, Bangladesh were retrospec- Open Access tively reviewed to identify all cases of Gynecologic malignancies and to de- termine the pattern of gynaecological malignancies identified between Janu- ary 2015 and December, 2018. -

Molar Pregnancy and Other Gestational Trophoblastic Diseases
27 Molar Pregnancy and other Gestational Trophoblastic Diseases Heleen van Beekhuizen INTRODUCTION chromo some pattern. Often a fetus or fetal tissue is present. Only 0.5–2% of partial moles develop into Gestational trophoblastic disease (GTD) is a placen- invasive moles1. tal disease: it arises from abnormal proliferation of trophoblastic cells in the placenta. When GTD INCIDENCE persists or recurs it is often called gestational tropho- blastic neoplasm (GTN). The spectrum of GTD Molar pregnancies are rare: approximately 1 in includes: every 400–800 pregnancies is a complete or partial • Complete and partial hydatidiform molar preg- molar pregnancy. Choriocarcinoma and persistent nancies: the most common form of GTD inva- trophoblastic neoplasm are even rarer with an sive mole (GTN). incid ence of approximately 1 : 50,000 pregnancies. • Choriocarcinoma, placental site trophoblastic Risk factors for GTD are: tumor (PSTT) and epithelioid trophoblastic • Teenage pregnancies tumor (ETT) which are all malignant degenera- • Pregnancies in women above the age of 35 years tions of placental tissue. Very rarely no ante cedent • History of molar pregnancy: the risk of recur- pregnancy can be identified. PSTT and ETT are rence is 1% after one molar pregnancy and rare and are not discussed further in this book. 15–20% after two molar pregnancies1 Most, but not all GTD produce β-human chorio- • Smoking nic gonadotropin (hCG), which is also produced in • Blood group A, B or AB normal pregnancies and can be detected with a • Nulliparity urine pregnancy test (UPT). • Use of oral contraceptives (no higher chance in persistent/invasive mole) Complete mole • History of infertility. -
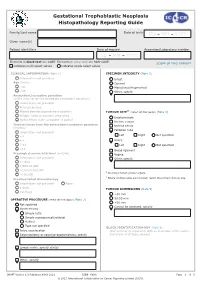
Proforma for Histopathology Reporting
Gestational Trophoblastic Neoplasia Histopathology Reporting Guide Family/Last name Date of birth DD – MM – YYYY Given name(s) Patient identifiers Date of request Accession/Laboratory number DD – MM – YYYY Elements in black text are CORE. Elements in grey text are NON-CORE. SCOPE OF THIS DATASET indicates multi-select values indicates single select values CLINICAL INFORMATION (Note 1) SPECIMEN INTEGRITY (Note 3) Information not provided Intact Age (years) Opened <40 Morcellated/fragmented ≥40 Other, specify Antecedent/causative gestation (which may not be the immediate antecedent gestation) Information not provided Term pregnancy a,b Missed abortion/spontaneous abortion TUMOUR SITE (select all that apply) (Note 4) Ectopic (tubal or ovarian) pregnancy Indeterminate Hydatidiform mole (complete or partial) Uterine corpus Interval times from the antecedent/causative gestation Uterine cervix (months) Fallopian tube Information not provided Left Right Not specified <4 4-6 Ovary 7-12 Left Right Not specified >12 Broad ligament Presurgical serum hCG level (mIU/ml) Vagina Information not provided Other, specify <1,000 1,000-10,000 >10,000-100,000 a Involving female genital organs. >100,000 b Previous failed chemotherapy Where multiple sites are involved, report the primary tumour site. Information not provided None 1 drug TUMOUR DIMENSIONS (Note 5) ≥2 drugs <30 mm 30-50 mm OPERATIVE PROCEDURE (select all that apply) (Note 2) >50 mm Not specified Cannot be assessed, specify Hysterectomy Simple total Simple supracervical/subtotal Radical Type not specified BLOCK IDENTIFICATION KEY (Note 6) Pelvic exenteration (List overleaf or separately with an indication of the nature Salpingectomy or salpingo-oophorectomy, specify and origin of all tissue blocks) Lymph nodes, specify site(s) Other, specify DRAFT Version 1.0 Published XXXX 2021 ISBN: XXXX Page 1 of 3 © 2021 International Collaboration on Cancer Reporting Limited (ICCR). -

Using Short Tandem Repeat Analysis for Choriocarcinoma Diagnosis: a Case Series Xiaofei Zhang1, Kai Yan2, Jianhua Chen1 and Xing Xie3*
Zhang et al. Diagnostic Pathology (2019) 14:93 https://doi.org/10.1186/s13000-019-0866-5 RESEARCH Open Access Using short tandem repeat analysis for choriocarcinoma diagnosis: a case series Xiaofei Zhang1, Kai Yan2, Jianhua Chen1 and Xing Xie3* Abstract Background: Choriocarcinoma is a highly aggressive, malignant trophoblastic neoplasm that can be gestational or non-gestational in origin. Accurate discrimination between these two subtypes, the causative pregnancy type, and the pregnancy-to-treatment interval for gestational choriocarcinoma are vital for clinical management. Methods: Fifteen choriocarcinomas were genotyped using multiplex fluorescent polymerase chain reaction amplification of 15 short tandem repeat (STR) loci and the amelogenin locus (XY determination). Genotype patterns at each locus from tumoral and maternal tissues were compared, and any prior or concurrent mole/placenta was also compared when available. According to STR results showing the presence or absence of the paternal chromosomal complement, the gestational or non-gestational origin of the tumor and the nature of the causative pregnancy was identified. Results: Fourteen tumors were gestational. Of these, seven were androgenetic/homozygous XX, and two were androgenetic/heterozygous XX, indicating that the causative pregnancies were molar pregnancies. Among the nine molar pregnancies, five were of the occult type. A menopausal patient developed a tumor from a mole that occurred seven years ago, identified by the genetically identical allele from the tumor and prior mole. One tumor originating from a previous mole was interrupted by term delivery. Two tumors found eight weeks postpartum were identified as originating from a prior occult mole. A pelvic choriocarcinoma was separated from a genetically distinct third trimester intrauterine placenta. -

Case Report Gestational Intraplacental Choriocarcinoma in a Term Pregnancy and Delivery: a Case Report and Review of the Literature
Int J Clin Exp Med 2016;9(3):6868-6872 www.ijcem.com /ISSN:1940-5901/IJCEM0017446 Case Report Gestational intraplacental choriocarcinoma in a term pregnancy and delivery: a case report and review of the literature Yan Chen1, Zhiguo Hu1, Yu Zhang2, Qiuliang Wu2 1Department of Pathology, Si’an Hospital of Guangdong Province, Dongguan, Guangdong Province, China; 2Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong Province, China Received October 8, 2015; Accepted December 29, 2015; Epub March 15, 2016; Published March 30, 2016 Abstract: Introduction: Choriocarcinoma is a rare but highly malignant trophoblastic neoplasm. Cases of its coex- istence with or after a “normal” pregnancy are extremely rare. Intraplacental choriocarcinoma is rare, and usually results in maternal metastasis at the time of diagnosis. Case presentation: We present the case of a 29 year-old gravida 1 para 0 Chinese woman who delivered a viable 3,140 g female infant at 38 weeks’ gestation. Because of the patient’s history of gestational diabetes mellitus and hepatitis B positive status, the placenta examined pathologically, and placental choriocarcinoma was diagnosed. The patient denied any previous pregnancies. Her serum beta human chorionic gonadotropin was 3573.7 mIU/ml 4 days after cesareansection, and dropped to less than 5 mIU/ml six weeks post-partum. There were no signs of dissemination; therefore, the patient received one course of chemotherapy. To date, both mother and baby are well. Conclusion: We postulate that the prevalence of intraplacental choriocarcinoma is notably higher than previously reported and remains undetected because it is not routine practice to send placentas for pathological evaluation after a normal spontaneous delivery. -

ESUR Quick Guide to Female Pelvis Imaging European Society of Urogenital Radiology
ESUR Quick Guide to Female Pelvis Imaging European Society of Urogenital Radiology 1.0 ESUR Quick Guide to Female Pelvis Imaging 1.0 PREFACE The concept of this booklet is to be a guide used as a quick reference to planning the imaging of patients with suspected or confirmed gynaecologic disease. Full guidelines for many of these disease areas are published. Here we hope to provide a quick and easily accessible reference for the radiologist and radiographers undertaking these imaging investigations. Comments and questions are welcome at [email protected] ESUR Female Pelvis Imaging Working Group April 2019 PREFACE 1 AUTHOR ACKNOWLEDGEMENTS Special thanks to the following authors who have contributed to the production of this Quick Guide booklet: • Celine Alt • Nishat Bharwani • Laura Brunesch • Francesco M. Danza • Martina Derme • Rania Farouk El Sayed • Rosemarie Forstner • Laure Fournier • Benedetta Gui • Aki Kido • Rahel A. Kubik-Huch • Rita Lucas • Teresa Margarida Cunha • Gabriele Masselli • Olivera Nikolic • Stephanie Nougaret • Milagros Otero-Garcia • Andrea Rockall • Evis Sala 2 AUTHOR Acknowledgements CONTENTS TERMINOLOGY: CONTRAST AGENTS AND CONTRAST MEDIA PREFACE 1 AUTHOR ACKNOWLEDGEMENTS 2 CONTENTS 3 1. TECHNICAL INFORMATION 4 2. DISEASE SPECIFIC INVESTIGATIONS 12 a. Cervical Cancer 12 i. Primary Staging 12 ii. Follow up and Investigation of Recurrence 16 b. Endometrial Cancer 19 c. Vaginal Masses 25 d. Characterisation of Adnexal Mass 28 e. Staging and Follow-up of Ovarian Cancer 32 f. Endometriosis 38 g. Leiomyoma 45 h. Female Tract Congenital Abnormalities 51 i. Gynaecologic Emergencies 55 j. Pelvic Floor Imaging 61 k. Placental Disease 65 l. Fistula 75 AUTHOR Acknowledgements CONTENTS 3 1.