Conservation Implications of Behavioural Interactions Between the Giant African Snail and a Native Brazilian Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Análisis Morfológico Del Sistema Reproductor E Identificación
Revista Peruana de Biología ISSN: 1561-0837 [email protected] Universidad Nacional Mayor de San Marcos Perú Jaramillo Roldán, Erika; López Martínez, Jessika; Ramírez, Rina; Velásquez Trujillo, Luz Elena Análisis morfológico del sistema reproductor e identificación molecular a través de los marcadores mitocondriales COI y 16S rRNA de Megalobulimus oblongus (Mollusca, Strophocheilidae) de Colombia Revista Peruana de Biología, vol. 21, núm. 1, mayo-, 2014, pp. 79-88 Universidad Nacional Mayor de San Marcos Lima, Perú Disponible en: http://www.redalyc.org/articulo.oa?id=195031025004 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Revista peruana de biología 21(1): 079 - 088 (2014) ISSN-L 1561-0837 Identificación morfológica y molecular de MEGALOBULIMUS OBLONGUS DE COLOMBIA doi: http://doi.org/10.15381/rpb.v21i1.8250 FACULTAD DE CIENCIAS BIOLÓGICAS UNMSM TRABAJOS ORIGINALES Análisis morfológico del sistema reproductor e identificación molecular a través de los marcadores mitocondriales COI y 16S rRNA de Megalobulimus oblongus (Mollusca, Strophocheilidae) de Colombia Morphological analysis of the reproductive system and molecular identification by mitochondrial markers COI and 16S rRNA of Megalobulimus oblongus (Mollusca, Strophocheilidae) of Colombia Erika Jaramillo Roldán1,3, Jessika López Martínez1,3, Rina Ramírez2, Luz Elena Velásquez Trujillo1,3 1 Grupo de Microbiología Ambiental, Escuela de Resumen Microbiología, Universidad de Antioquia, Medel- lín, Colombia. En este trabajo se hizo el análisis morfológico y molecular a 28 caracoles terrestres de Mega- 2 Laboratorio de Sistemática Molecular y Filogeo- lobulimus oblongus, colectados en diferentes departamentos de Colombia, depositados en grafía, Facultad de Ciencias Biológicas; y Museo una colección de referencia. -

December 2011
Ellipsaria Vol. 13 - No. 4 December 2011 Newsletter of the Freshwater Mollusk Conservation Society Volume 13 – Number 4 December 2011 FMCS 2012 WORKSHOP: Incorporating Environmental Flows, 2012 Workshop 1 Climate Change, and Ecosystem Services into Freshwater Mussel Society News 2 Conservation and Management April 19 & 20, 2012 Holiday Inn- Athens, Georgia Announcements 5 The FMCS 2012 Workshop will be held on April 19 and 20, 2012, at the Holiday Inn, 197 E. Broad Street, in Athens, Georgia, USA. The topic of the workshop is Recent “Incorporating Environmental Flows, Climate Change, and Publications 8 Ecosystem Services into Freshwater Mussel Conservation and Management”. Morning and afternoon sessions on Thursday will address science, policy, and legal issues Upcoming related to establishing and maintaining environmental flow recommendations for mussels. The session on Friday Meetings 8 morning will consider how to incorporate climate change into freshwater mussel conservation; talks will range from an overview of national and regional activities to local case Contributed studies. The Friday afternoon session will cover the Articles 9 emerging science of “Ecosystem Services” and how this can be used in estimating the value of mussel conservation. There will be a combined student poster FMCS Officers 47 session and social on Thursday evening. A block of rooms will be available at the Holiday Inn, Athens at the government rate of $91 per night. In FMCS Committees 48 addition, there are numerous other hotels in the vicinity. More information on Athens can be found at: http://www.visitathensga.com/ Parting Shot 49 Registration and more details about the workshop will be available by mid-December on the FMCS website (http://molluskconservation.org/index.html). -

Sôbre a Ocorrência De Strophocheilidae (Molusco Gastrópode) No Rio Grande Do Sul
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cadernos Espinosanos (E-Journal) SÔBRE A OCORRÊNCIA DE STROPHOCHEILIDAE (MOLUSCO GASTRÓPODE) NO RIO GRANDE DO SUL PAULO SAWAYA e JORGE ALBERTO PETERSEN* (Departamento de Fisiologia Geral e Animal da USP, e Instituto de Ciências Naturais da Univ. R. G. do Sul) (Com 1 Est.) O presente trabalho teve por origem a necessidade de determi nar a espécie dos caracóis coletados para execícios práticos de fi siologia comparada. Os caracteres dos animais do nosso material coincidem em grande parte com os de Strophocheilus oblongus mus- culus Becquaert, 1948, subespécie até agora somente citada para a Argentina e Paraguai. Para o nosso estudo seguimos a monografia elaborada por Bec quaert (1948) sôbre a revisão da família Strophocheilidae. Dela nos servimos também para a discussão e comentários. Grande auxílio nos prestou a excelente coleção malacológica existente no Departamento de Zoologia da Secretaria de Agricultura de São Paulo, estudada pelo próprio Becquaert. Agradecemos ao Dr. Celso Paulo Jaeger o fornecimento de vários caracóis; ao Dr. Lindolpho Guimarães e ao Lic. José Luiz Moraes Le me também agradecemos pelas facilidades proporcionadas para a con sulta da coleção malacológica do Departamento de Zoologia da Se cretaria de Agricultura do Estado de São Paulo. Daremos a seguir a diagnose do gênero, do subgênero e os ele mentos de que nos valemos para a determinação da subespécie, e após a discussão, apresentaremos alguns comentários sôbre as Stropho cheilidae . * Bolsista da Universidade de São Paulo. 32 P. SAWAYA & J. A. PETER SEN GÊNERO STROPHOCHEILUS SPIX, 1827 Caracteres da concha: tamanho médio a muito grande (20 a 160 mm de comprimento), grande capacidade, oblonga-ovalada, elíptica ou em forma de fuso, relativamente larga em relação ao comprimento, sendo porém êste sempre maior do que a largura, com uma volta do corpo bastante desenvolvida, e com um ápice obtuso arredondado. -

Threatened Freshwater and Terrestrial Molluscs
Biodiversity Journal, 2011, 2 (2): 59-66 Threatened freshwater and terrestrial molluscs (Mollusca, Gastropoda et Bivalvia) of Santa Catarina State, Southern Brazil: check list and evaluation of regional threats A. Ignacio Agudo-Padrón Project “Avulsos Malacológicos”, Caixa Postal (P.O. Box) 010, 88010-970, Centro, Florianópolis, Santa Catarina, SC, Brasil; [email protected]; http://www.malacologia.com.br ABSTRACT A total of nineteen continental native mollusc species are confirmed for the Santa Catarina State (SC) (organized in ten Genera and seven Families), one aquatic Prosobranchia/Caenogastropoda (Ampullariidae), six Pulmonata terrestrial gastropods (one Ellobiidae, three Megalobulimidae and two micro-snails – Charopidae and Streptaxidae) and twelve freshwater mussels (eight Mycetopodidae and four Hyriidae). These species are designated by the International Union for Conservation of the Nature – IUCN as follows: seven as "Vulnerable", six "In Danger" and six “Without Category Established”. The general regional threats that these species are subjected to are briefly analyzed. KEY WORDS Biodiversity, Continental mollusc fauna, Threatened species, Santa Catarina State, Southern Brazil region Received 18.02.2011; accepted 12.04.2011; printed 30.06.2011 INTRODUCTION access is quite restricted and permitted only to researchers; this besides four “National In spite of prodigious scientific and Ecological Parks” within the jurisdiction of the technological progress in recent years, in same State. throughout Brazil and other Neotropical -

Land Snail Diversity in Brazil
2019 25 1-2 jan.-dez. July 20 2019 September 13 2019 Strombus 25(1-2), 10-20, 2019 www.conchasbrasil.org.br/strombus Copyright © 2019 Conquiliologistas do Brasil Land snail diversity in Brazil Rodrigo B. Salvador Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand. E-mail: [email protected] Salvador R.B. (2019) Land snail diversity in Brazil. Strombus 25(1–2): 10–20. Abstract: Brazil is a megadiverse country for many (if not most) animal taxa, harboring a signifi- cant portion of Earth’s biodiversity. Still, the Brazilian land snail fauna is not that diverse at first sight, comprising around 700 native species. Most of these species were described by European and North American naturalists based on material obtained during 19th-century expeditions. Ear- ly 20th century malacologists, like Philadelphia-based Henry A. Pilsbry (1862–1957), also made remarkable contributions to the study of land snails in the country. From that point onwards, however, there was relatively little interest in Brazilian land snails until very recently. The last de- cade sparked a renewed enthusiasm in this branch of malacology, and over 50 new Brazilian spe- cies were revealed. An astounding portion of the known species (circa 45%) presently belongs to the superfamily Orthalicoidea, a group of mostly tree snails with typically large and colorful shells. It has thus been argued that the missing majority would be comprised of inconspicuous microgastropods that live in the undergrowth. In fact, several of the species discovered in the last decade belong to these “low-profile” groups and many come from scarcely studied regions or environments, such as caverns and islands. -

Revised and Updated Systematic Inventory of Non-Marine Molluscs
Agudo-Padron. Advances Environ Stud 2018, 2(1):54-60 DOI: 10.36959/742/202 | Volume 2 | Issue 1 Advances in Environmental Studies Review Article Open Access Revised and Updated Systematic Inventory of Non-Marine Molluscs Occurring in the State of Santa Catarina/SC, Cen- tral Southern Brazil Region A Ignacio Agudo-Padron* Researcher Malacologist, Avulsos Malacológicos - AM, Santa Catarina State, Brazil Abstract Based on the last list of non-marine molluscs from Santa Catarina state, published in 2014, the current inventory of conti- nental molluscs (terrestrial and freshwater) occurring in the State of Santa Catarina/SC is finally consolidated, with a veri- fied/confirmed registry of 232 species and subspecies, sustained product of complete 22 years of systematic field researches, examination of specimens deposited in collections of museums and parallel reference studies, covering 198 gastropods (156 terrestrial, 2 amphibians, 40 freshwater) and 34 limnic bivalves, in addition to the addition of another new twelve (12) species (eighth land gastropods - Leptinaria parana (Pilsbry, 1906); Bulimulus cf. stilbe Pilsbry, 1901; Orthalicus aff. prototypus (Pilsbry, 1899); Megalobulimus abbreviatus Bequaert, 1848; Megalobulimus januarunensis Fontanelle, Cavallari & Simone, 2014; Megalobulimus sanctipauli (Ihering, 1900); Happia sp (in determination process); Macrochlamys indica Benson, 1832 - and four bivalves - Corbicula fluminalis (Müller, 1774); Pisidium aff. dorbignyi (Clessin, 1879); Pisidium aff. vile (Pilsbry, 1897); Sphaerium cambaraense -
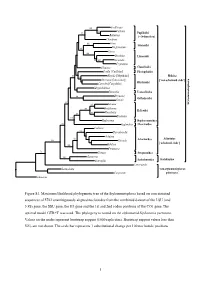
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Snail and Slug Dissection Tutorial: Many Terrestrial Gastropods Cannot Be
IDENTIFICATION OF AGRICULTURALLY IMPORTANT MOLLUSCS TO THE U.S. AND OBSERVATIONS ON SELECT FLORIDA SPECIES By JODI WHITE-MCLEAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2012 1 © 2012 Jodi White-McLean 2 To my wonderful husband Steve whose love and support helped me to complete this work. I also dedicate this work to my beautiful daughter Sidni who remains the sunshine in my life. 3 ACKNOWLEDGMENTS I would like to express my sincere gratitude to my committee chairman, Dr. John Capinera for his endless support and guidance. His invaluable effort to encourage critical thinking is greatly appreciated. I would also like to thank my supervisory committee (Dr. Amanda Hodges, Dr. Catharine Mannion, Dr. Gustav Paulay and John Slapcinsky) for their guidance in completing this work. I would like to thank Terrence Walters, Matthew Trice and Amanda Redford form the United States Department of Agriculture - Animal and Plant Health Inspection Service - Plant Protection and Quarantine (USDA-APHIS-PPQ) for providing me with financial and technical assistance. This degree would not have been possible without their help. I also would like to thank John Slapcinsky and the staff as the Florida Museum of Natural History for making their collections and services available and accessible. I also would like to thank Dr. Jennifer Gillett-Kaufman for her assistance in the collection of the fungi used in this dissertation. I am truly grateful for the time that both Dr. Gillett-Kaufman and Dr. -

A Review on Persisting Threats to Snail's Diversity and Its Conservation Approaches
Archives of Agriculture and Environmental Science 5(2): 205-217 (2020) https://doi.org/10.26832/24566632.2020.0502019 This content is available online at AESA Archives of Agriculture and Environmental Science Journal homepage: journals.aesacademy.org/index.php/aaes e-ISSN: 2456-6632 REVIEW ARTICLE A review on persisting threats to snail’s diversity and its conservation approaches Varun Dhiman1* , Deepak Pant2, Dushyant Kumar Sharma3 and Prem Prakash4 1Waste Management Laboratory, School of Earth & Environmental Sciences, Central University of Himachal Pradesh, Shahpur - 176206 (Himachal Pradesh), INDIA 2School of Chemical Sciences, Central University of Haryana, Jant-Pali, Mahendergarh, Haryana-123031, INDIA 3Department of Tree improvement and Genetic resources, College of Horticulture and Forestry, Neri, Hamirpur (Himachal Pradesh) – 177001, INDIA 4Department of Silviculture and Agroforestry, College of Horticulture and Forestry, Neri, Hamirpur (Himachal Pradesh) – 177001, INDIA *Corresponding author’s E-mail: [email protected] ARTICLE HISTORY ABSTRACT Received: 24 March 2020 Snails are important part of our terrestrial, freshwater and marine ecosystems. They are Revised received: 15 May 2020 molluscian members having their effective role in biomonitoring, nutrient cycling and medici- Accepted: 26 May 2020 nal development. They are integral part of food chain system and have direct or indirect impact in maintaining ecological functioning. Besides their importance, they are documented with largest extinction rate as compared to other existed taxa. This is due to the fact that, Keywords unexploration and poor attention has been given in the research and development by the scientific world. Till now, a little information is available related to systematics, life history, Conservation Diversity population biology, threats and conservation status of these slimy organisms. -

December 2006
("% $##& $##' ! $##' &)+4%)*&% +-4#*++ 44 *% ## )- ><=&,+##%+)+1, +>== <C=; ')") - ++&##1<AC;< %&/- ##1>BC=C C<?5=>?5?;D; CA@5@?@5?<?;/<B/3??DA &)+88%)*&%:.*4&- #*++:,**4&- +) &)) *&% 4,%% 1 & -) *#%* &#& #' # *+* %4 44&/<C<< <?<B & %,*+) #)" )")*,)1=A<;= 9##&%1A>>AA >;?5?==5;B@= A>A5=C<5<DC=/3;DB> '+) 8$&)) *&%:.*4&- ,%%:&#& #*' # *+*4&$ 4&$*++)* ,*,$& &#& # -)* +0 &++% -)* +0 <><@ %%)& &#,$,*1 ?>=<= A<?5=D=5A<B; ++)*4<:&*,4, ) *+ %0) ## %& *+,)# *+&)0,)-0 <C<A"+)+1$' %1 A<C=; $0): %*4, ,4, ,$ ** &%*&)+') #=;;B **,&## '*) $0*%++&+ +&)+%0+ $ ,+ ) , 0 4%0&%$0*,$ +%)+ #,+0&,$,*+ $$)&+&) -## '*) 4#*# $ +*,$ ** &%* +& &,+ &% '4 +&) *&)&%+) ,+ &%* %#,%.*1%.',# + &%*1$+ %%%&,%$%+*1 ,))%+ **,*+ %$&##,*"*1!&'&*+ %*1&%+) ,+)+ #*6 %#, %&%& % )*) ')&!+*71 *+)+*1 %*& +0&$$ ++)'&)+*4#+)&% *,$ ** &%* )')))2&%+++ +&). +%0(,*+ &%*4&+++*,$ ** &%*)%&+') )- .1,+)"&)&%+%+%%)# + %4 %"*+& )$0 $%%&)#'**$# %%$ # %+ *%.*#++)4 #**%%&)** %&)$+ &%+&+)+)01++0&)) *&%4 NEWSLETTER OF THE FRESHWATER MOLLUSK CONSERVATION SOCIETY ) % !! ! ! $""( # & ' ' ! #' FMCS 2007 SYMPOSIUM & WORKSHOP MARCH 11 – 15, 2007 THE PEABODY LITTLE ROCK LITTLE ROCK, ARKANSAS Join us for the 5th Biennial Symposium of the Freshwater Mollusk Conservation Society, to be held at The Peabody Little Rock hotel in Little Rock, Arkansas from March 13-15, 2007. A one-day FMCS sponsored workshop on Habitat Restoration will be conducted prior to the symposium on Monday, March 12. The symposium theme is Directions in -

Moluscos Terrestres Do Brasil (Gastrópodes Operculados Ou Não, Exclusive Veronicellidae, Milacidae E Limacidae)1
Rev. Biol. Trop. 51 (Suppl. 3): 149-189, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Moluscos terrestres do Brasil (Gastrópodes operculados ou não, exclusive Veronicellidae, Milacidae e Limacidae)1 Norma Campos Salgado2 y Arnaldo C. dos Santos Coelho2 1 Contribuição 73, da Malacologia, Departamento de Invertebrados, Museu Nacional/Universidade Federal do Rio de Janeiro, RJ. Brasil 2 Departamento de Invertebrados, Museu Nacional, Quinta da Boa Vista, São Cristovão, 20940-040, Rio de Janeiro, RJ, Brasil; [email protected]; [email protected] Abstract: Studies on terrestrial prosobranchs (streptoneureans) and shelled pulmonates (euthyneureans) show the significant diversity of the Brazilian malacofauna. These mollusks are still poorly known, despite the increas- ing interest in the group that started in the XVIII century when land mollusks began to be collected and deposit- ed in scientific collections. The species are arranged in alphabetical order; the taxonomic combination is updat- ed when possible with the original and other bibliographic references. This checklist includes original study of specimens deposited in Brazilian, American and European collections, as well as names and additional data of important early naturalists and current researchers of the group. Species are here associated to their original ref- erences; geographical distribution and other taxonomic references were added to each. A total of 590 species were found (27 families and 95 genera). The systematic arrangement of suprageneric and generic taxa was based on Taylor & Sohl (1962), Thiele (1929-1931), Wenz (1938-1944) and Zilch (1959-1960). Breure (1973-1985) was especially useful regarding Bulimuloidea because the characteristics of some subgenera justified a raise to generic level. -

José Heitzmann Fontenelle São Paulo 2012
José Heitzmann Fontenelle ANATOMIA, TAXONOMIA E DISTRIBUIÇÃO GEOGRÁFICA DOS CARAC ÓIS - GIGANTES DO “ C O M P L E X O MEGALOBULIMUS GRANUL OSUS ” (MOLLUSCA, GASTROPODA, PULMONATA ) São Paulo 2012 José Heitzmann Fontenelle ANATOMIA, TAXONOMIA E DISTRIBUIÇÃO GEOGRÁFICA DOS CARACÓIS-GIGANTES DO “COMPLEXO MEGALOBULIMUS GRANULOSUS” (MOLLUSCA, GASTROPODA, PULMONATA) ANATOMY, TAXONOMY AND GEOGRAPHICAL DISTRIBUTION OF THE GIANT SNAILS “COMPLEX MEGALOBULIMUS GRANULOSUS” (MOLLUSCA, GASTROPODA, PULMONATA) Dissertação apresentada ao Instituto de Biociências da Universidade de São Paulo, para a obtenção de Título de Mestre em Zoologia, na Área de Malacologia. Orientador: Prof. Dr. Luiz Ricardo Lopes de Simone São Paulo 2012 Heitzmann Fontenelle, José Anatomia, taxonomia e distribuição geográfica dos caracóis gigantes do “Complexo Megalobulimus granulosus” (Mollusca, Gastropoda, Pulmonata). VIII, 160 p. Dissertação (Mestrado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Zoologia. 1. “complexo Megalobulimus granulosus” 2. anatomia comparada 3. taxonomia 4. distribuição geográfica I. Universidade de São Paulo. Instituto de Biociências. Departamento de Zoologia. Comissão Julgadora: ________________________ _______________________ Prof(a). Dr(a). Prof(a). Dr(a). ______________________ Prof. Dr. Luiz Ricardo Lopes de Simone Orientador(a) ÍNDICE 1) INTRODUÇÃO 1.1) Biologia dos Megalobulimus....................................................................................... 1 1.2) Histórico taxonômico..................................................................................................