Identification of Potential Environmental Impacts on Surface Water Associated with Cranberry Development in Newfoundland And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
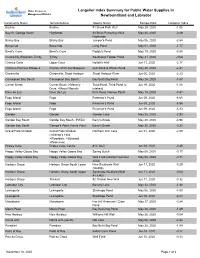
Langelier Index Summary for Public Water Supplies in Newfoundland
Water Resources Langelier Index Summary for Public Water Supplies in Management Division Newfoundland and Labrador Community Name Serviced Area Source Name Sample Date Langelier Index Bauline Bauline #1 Brook Path Well May 29, 2020 -1.06 Bay St. George South Highlands #3 Brian Pumphrey Well May 20, 2020 -0.29 Highlands Birchy Bay Birchy Bay Jumper's Pond May 06, 2020 -2.64 Bonavista Bonavista Long Pond May 01, 2020 -1.77 Brent's Cove Brent's Cove Paddy's Pond May 19, 2020 -5.85 Centreville-Wareham-Trinity Trinity Southwest Feeder Pond May 21, 2020 -3.64 Chance Cove Upper Cove Hollett's Well Jun 11, 2020 -2.17 Channel-Port aux Basques Channel-Port Aux Basques Gull Pond & Wilcox Pond May 20, 2020 -2.41 Clarenville Clarenville, Shoal Harbour Shoal Harbour River Jun 05, 2020 -2.23 Conception Bay South Conception Bay South Bay Bulls Big Pond May 28, 2020 -1.87 Corner Brook Corner Brook (+Massey Trout Pond, Third Pond (2 Jun 19, 2020 -1.88 Drive, +Mount Moriah) intakes) Fleur de Lys Fleur De Lys First Pond, Narrow Pond May 19, 2020 -3.41 Fogo Island Fogo Freeman's Pond Jun 09, 2020 -6.65 Fogo Island Fogo Freeman's Pond Jun 09, 2020 -6.54 Fogo Island Fogo Freeman's Pond Jun 09, 2020 -6.33 Gander Gander Gander Lake May 25, 2020 -2.93 Gander Bay South Gander Bay South - PWDU Barry's Brook May 20, 2020 -4.98 Gander Bay South George's Point, Harris Point Barry's Brook May 20, 2020 -3.25 Grand Falls-Windsor Grand Falls-Windsor Northern Arm Lake Jun 01, 2020 -2.80 (+Bishop's Falls, +Wooddale, +Botwood, +Peterview) Grates Cove Grates Cove Centre #1C -

Central Newfoundland Solid Waste Management Plan
CENTRAL NEWFOUNDLAND SOLID WASTE MANAGEMENT PLAN Phase I Report Volume I Final Report Submitted to Central Newfoundland Waste Management Committee BNG PROJECT # 721947 Fogo Crow Head Glovers Harbour New-Wes-Valley Wooddale Badger Gander Grand Falls-Windsor Salvage Buchans CENTRAL NEWFOUNDLAND SOLID WASTE MANAGEMENT PLAN Phase I Report Final Report Submitted to: Central Newfoundland Waste Management Committee c/o Town of Gander P.O. Box 280 Gander, NF A1V 1W6 Submitted by: BAE-Newplan Group Limited 1133 Topsail Road, Mount Pearl Newfoundland, Canada A1N 5G2 October, 2002 CENTRAL NEWFOUNDLAND SOLID WASTE MANAGEMENT PLAN Phase I Report Project No.: 721947 Title: CENTRAL NEWFOUNDLAND SOLID WASTE MANAGEMENT PLAN Phase I Report - Final Client: Central Newfoundland Waste Management Committee C 02/10/02 Final Report GW WM WM B 02/09/18 Final Draft Report GW/ZY WM WM A 02/06/05 Issued for Review GW/PH/ZY WM WM Rev. Date Page No. Description Prepared By Reviewed Approved yyyy/mm/dd By By CENTRAL NEWFOUNDLAND SOLID WASTE MANAGEMENT PLAN Page i Phase I Report TABLE OF CONTENTS 1.0 INTRODUCTION.............................................................................................................1 1.1 Background and Objectives.........................................................................................1 2.0 STUDY AREA BOUNDARY ...........................................................................................3 3.0 POPULATION PROJECTION.........................................................................................6 -

Office Allowances - Office Accommodations 01-Apr-18 to 31-Mar-19
House of Assembly Newfoundland and Labrador Member Accountability and Disclosure Report Office Allowances - Office Accommodations 01-Apr-18 to 31-Mar-19 Dean, Jerry, MHA Page: 1 of 1 Summary of Transactions Processed to Date for Fiscal 2018/19 Expenditure Limit (Net of HST): $19,200.00 Transactions Processed as of: 31-Mar-19 Expenditures Processed to Date (Net of HST): $19,200.00 Funds Available (Net of HST): $0.00 Percent of Funds Expended to Date: 100.0% Date Source Document # Vendor Name Expenditure Details Amount 01-Apr-18 HOA004815 W REID CONSTRUCTION Lease payment for the Constituency Office of the MHA for the District of Exploits 1,600.00 LTD located in Bishop's Falls. 01-May-18 HOA004871 W REID CONSTRUCTION Lease payment for the Constituency Office of the MHA for the District of Exploits 1,600.00 LTD located in Bishop's Falls. 01-Jun-18 HOA004908 W REID CONSTRUCTION Lease payment for the Constituency Office of the MHA for the District of Exploits 1,600.00 LTD located in Bishop's Falls. 01-Jul-18 HOA004946 W REID CONSTRUCTION Lease payment for the Constituency Office of the MHA for the District of Exploits 1,600.00 LTD located in Bishop's Falls. 01-Aug-18 HOA004983 W REID CONSTRUCTION Lease payment for the Constituency Office of the MHA for the District of Exploits 1,600.00 LTD located in Bishop's Falls. 01-Sep-18 HOA005021 W REID CONSTRUCTION Lease payment for the Constituency Office of the MHA for the District of Exploits 1,600.00 LTD located in Bishop's Falls. -

Mos Ratification Vote Schedule 2018
MOS RATIFICATION VOTE SCHEDULE 2018 DATE TIME WORK LOCATION MEETING LOCATION Monday, Jan 8 9:00 a.m. White Hills Depot Monday, Jan 8 11:00 a.m. Donovans Depot Monday, Jan 8 1:30 p.m. Foxtrap Depot Monday, Jan 8 7:30 p.m. Local 7308 Holiday Inn Highway Enforcement Tuesday, Jan 9 9:00 a.m. Avondale Depot Tuesday, Jan 9 11:30 a.m. Whitbourne Depot Tuesday, Jan 9 2:00 p.m. Goobies Depot Tuesday, Jan 9 7:30 p.m. Clarenville Clarenville Inn Lethbridge Highway Enforcement Public Works MOS Engineering Wednesday, Jan 10 9:00 a.m. Port Rexton Depot Wednesday, Jan 10 11:00 a.m. Amherest Cove Depot Monday, Jan 15 11:00 a.m. Eastporte Eastporte Depot Grant=s Pit Charletown St. Brendan=s Monday, Jan 15 10:00 a.m. Lumsden Depot Monday, Jan 15 1:30 p.m. New World Island Depot Monday, Jan 15 7:30 p.m. Grand Falls Mount Peyton Hotel Bishops Falls Northern Arm Public Works Wooddale Tree Nursery Highway Enforcement MOS Engineering Tuesday, Jan 16 11:00 a.m. Baie Verte Baie Verte Depot LaScie Public Works Tuesday, Jan 16 11:00 a.m. Sops Arm Sops Arm Depot Hampton Depot Tuesday, Jan 16 7:00 p.m. Deer Lake Holiday Inn Express Glenburnie Public works Highway Enforcement MOS Engineering Wednesday, Jan 17 1:30 p.m. St. Anthony Depot Wednesday, Jan 17 11:00 a.m. Port aux Basques St. Christopher=s Hotel Wednesday, Jan 17 1:00 p.m. Doyles Depot Wednesday, Jan 17 4:00 p.m. -

Community Files in the Centre for Newfoundland Studies
Community Files in the Centre for Newfoundland Studies A | B | C | D | E | F | G | H | I | J | K | L | M | N | 0 | P | Q-R | S | T | U-V | W | X-Y-Z A Abraham's Cove Adams Cove, Conception Bay Adeytown, Trinity Bay Admiral's Beach Admiral's Cove see Port Kirwan Aguathuna Alexander Bay Allan’s Island Amherst Cove Anchor Point Anderson’s Cove Angel's Cove Antelope Tickle, Labrador Appleton Aquaforte Argentia Arnold's Cove Aspen, Random Island Aspen Cove, Notre Dame Bay Aspey Brook, Random Island Atlantic Provinces Avalon Peninsula Avalon Wilderness Reserve see Wilderness Areas - Avalon Wilderness Reserve Avondale B (top) Baccalieu see V.F. Wilderness Areas - Baccalieu Island Bacon Cove Badger Badger's Quay Baie Verte Baie Verte Peninsula Baine Harbour Bar Haven Barachois Brook Bareneed Barr'd Harbour, Northern Peninsula Barr'd Islands Barrow Harbour Bartlett's Harbour Barton, Trinity Bay Battle Harbour Bauline Bauline East (Southern Shore) Bay Bulls Bay d'Espoir Bay de Verde Bay de Verde Peninsula Bay du Nord see V.F. Wilderness Areas Bay L'Argent Bay of Exploits Bay of Islands Bay Roberts Bay St. George Bayside see Twillingate Baytona The Beaches Beachside Beau Bois Beaumont, Long Island Beaumont Hamel, France Beaver Cove, Gander Bay Beckford, St. Mary's Bay Beer Cove, Great Northern Peninsula Bell Island (to end of 1989) (1990-1995) (1996-1999) (2000-2009) (2010- ) Bellburn's Belle Isle Belleoram Bellevue Benoit's Cove Benoit’s Siding Benton Bett’s Cove, Notre Dame Bay Bide Arm Big Barasway (Cape Shore) Big Barasway (near Burgeo) see -

Understanding Land Use in the Grand Falls-Windsor – Baie Verte – Harbour Breton Region Final Report
Understanding Land Use in the Grand Falls-Windsor – Baie Verte – Harbour Breton Region Final Report By Janelle Skeard, Maggie Sutherland, Patrick Leveque, and Kelly Vodden March 2013 1 Acknowledgements The authors and researchers would like to acknowledge the valuable participation and input from residents and stakeholders from, and associated with, the Grand Falls-Windsor – Baie Verte – Harbour Breton Region in this research project. This report would not be possible without their contributions, insights, and experiences. We would also like to acknowledge the support and assistance of the Grand Falls-Windsor- Baie Verte - Harbour Breton Regional Council and the Rural Secretariat Executive Council. It has been through their guidance, input, and experience that this project has succeeded. The researchers would like to give an extended thanks to Linda Brett, Regional Partnership Planner with the Rural Secretariat who has been instrumental in putting together this work. Her commitment, insights and patience on this project have been crucial and are very much appreciated. We are also grateful to Fiona Munro, Sarah Minnes, Heather Hall and Vesna Kerezi for their helpful comments and assistance. The case studies component of the project was made possible by the help and guidance of Geog3350 Instructor Ryan Gibson. Finally, we would like to thank our project partner, Municipalities Newfoundland and Labrador for their feedback and willingness to assist in ensuring valuable input was gained from municipal leaders in the region. The authors would -

November-29-2013-Independent-Engineer
315 kV HVac Transmission Lines Route Hopedale 4 Makkovik 4 Schefferville 4 Rigolet 4 Cartwright 4 North West River Churchill Falls 4 43& Happy Valley-Goose Bay 3& 4 Labrador City 4 Port Hope Simpson 4 Lourdes-de-Blanc-SablonSt. Anthony 4 4 National Geographic, Esri, DeLorme,Roddickton 4 Line 1: STR 323 X: 546099.372 E NAVTEQ, UNEP-WCMC, USGS,Port Saunders NASA, Y: 5896532.044 N 4 Line 2: STR 322 ESA, METI, NRCAN, GEBCO, NOAA, iPC X: 546119.152 E Y: 5896578.168 N KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKK 4 WEST CONTRACT EAST CONTRACT CHURCHILL FALLS SUBSTATION 3 MUSKRAT FALLS SWITCHYARD Accommodations Complex South Side Access Rd. 3 3 FUTURE GULL ISLAND INTERCONNECTION PROVISION FUTURE GULL ISLAND HYDRO DEVELOPMENT Ê 05 10203040 km Sources: Esri, DeLorme, NAVTEQ, TomTom, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), and the GIS User Community 350 kV HVdc Transmission Line Route ! ! Spruce! Park Batteau ! MF 138k/25kVMF 315kV/138kVp! Mud Lake ! ! Base des Forces canadiennes!.!. Goose Bay Canadian Forces Base Goose Bay Frenchmans Island Muskrat Falls ! ! Comfort Bight ! Cartwright Junction ! Hawke Harbour Terre-Neuve-et-Labrador ! ! C Newfoundland and Labrador ONS Norman's Bay Normans! Bay ! Happy Valley-Goose Bay 1 TR ! UCTUCTI ! Snug Harbour Triangle I ! ON ! Dead Islands Charlottetown SEGSEG ! ! Pensons Arm St. Anthony MEMEN ! ! N T 1 Fishing Ships Harbour T 1 Rexons Cove ! ! !! Port Hope Simpson -
Newfoundland Soil Survey and Land Use Program 1981
. ~ , ~._,.- ~.., NEWFOUNDLAND it -- SOIL SURVEY AND LAND USE PROGRAM 1981 The printing of this publication was funded jointly by the Canada/Newfoundland Agriculture Development Subsidiary Agreement. Copies of this publication may be obtained from : The Dept. of Rural, Agricultural and Northern Development, Soil and Land Management Division, Provincial Agriculture Building, Brookfield Rood, Mount Pearl, Newfoundland. AIC 5T7 NEWFOUNDLAND SOIL SURVEY AND LAND USE PROGRAM 1981 TABLE OF CONTENTS PAGE SOIL SURVEY PROGRAM 1 Exploratory Soil Surveys 3 Reconnaissance Soil Survey 7 Detailed Soil Surveys 10 LAND USE PROGRAM 14 Historical Background 14 Farm Land Preservation 15 Farm Land Development Projects 22 FIGURES Figure 1 Exploratory Soil Survey Program 6 Figure 2 Current Reconnaissance Soil Survey Program 8 Figure 3 Detailed Soil Surveys, Performance Management Surveys, Miscellaneous 13 Surveys Figure 4 Location of Arricultural Development Areas 17 Figure 5 Planning Process for Agricultural Development Areas 21 TABLES Table 1 Exploratory Soil Survey Program 5 Table 2 Current Reconnaissance Soil Survey Program 9 Table 3 Detailed Soil Surveys, Performance Management Surveys and Miscellaneous Surveys 12 NEWFOUNDLAND SOIL SURVEY AND LAND USE PROGRAM 1981 TABLE OF CONTENTS (con't) PAGE AGRICULTURAL DEVELOPMENT AREAS 1981 Map 1 ----- St. John's 25 Map 2 ----- Wooddale 26 Map 3 ----- Humber Valley 27 Map 4 ----- Robinson's - St. Fintan's 28 Map 5 ----- Codroy Valley 29 Map 6 ----- Musgravetown 30 Map 7 ----- Green Bay (Halls Bay-Springdale) 31 Map 8 ----- Green Bay (King's Point) 32 Map 9 ----- Green Bay (Indian Pond) 33 Map 10 ----- Port Au Port 34 Map 11 ----- Winter land 35 Map 12 ----- Burin 36 Map 13 ----- Markland 37 Map 14 ----- Lewisporte (Brown's Arm - Botwood) 38 Map 15 ----- Lewisporte (Comfort Cove) 39 Map 16 ----- Gander Lake 40 Map 17 ----- Terra Nova 41 Map 18 ----- Avalon South 42 Map 19 ----- Bay d'Espoir 43 SOIL SURVEY PROGRAM The Newfoundland landscape can be best described as a mixture of bogs, barrens, rock outcrops, water bodies and mineral soil. -

House of Assembly Hansard Speaker Index
HOUSE OF ASSEMBLY PROVINCE OF NEWFOLNDLAND 1991 3rd SESSION - 41st GENERAL ASSEMBLY HANSARD SPEAKER INDEX Published under the authority of the Speaker of the House of Assembly Honourable Thomas Lush HANSARD SPEAKER INDEX Aylward, Mr. Kevin (I, Stephenvllle) BURGEO and Port Saunders hospitals - engineering COLLEGES Act (Bill 37) contracts Second reading p. 3111-13 Newlab Engineering and Nycum and Associates EXECUTIVE Council Estimates - Committee of p. 1137-38;1165 Supply p. 1604-06 Table proposals p. 1245-46 INTERIM Supply CAPiTAL roads program Debate Bk. 16A, p. 15-17;19-22 List of projects p. 914 LOAN Act CO-OPERATIVE Societies Act (Bill 24) Debate p. 2134-35 Second reading p. 2052 MEDICARE program COLLECTIVE agreements Debate p. 2672-76 Meeting with unions p. 1699-1701 MEMORIAL University Extension Services closure COYNE, Deborah, former constitutional advisor Debate p. 1128-29 Salary p. 2900 NEWFOUNDLAND Hydro DEBATE on the Adjournment Source of power beyond year 2000; plant at Agri-Foods Task force Report p. 1690 Holyrood p. 1363-64 Engineering design contracts for Burgeo and ORAL Questions Port Saunders hospitals p. 1165 - Newfoundland Hydro - power source after year Royal Newfoundland Constabulary policing p. 2000; Holyrood plant p. 1363-64 981-82 SCHOOLS Act (Bill 31) Student employment program p. 665 Second reading p. 2124 DEPARTMENT of Works, Services and Transporta SOCIAL Services Estimates Committee (Concur tion rence Motion) Number of positions eliminated p. 3 13-14 Debate p. 2090-92 DRIVER’S licence and vehicle permit renewal ap SUPPLEMENTARY Supply, No. 3 plications Debate p. 2425-27 Changes p. 1170 TOBACCO Tax DUMP truck owner/operators Debate p. -

The Pre-Industrial Condition of the Forest Limits of Corner Brook Pulp and Paper Limited
THE PRE-INDUSTRIAL CONDITION OF THE FOREST LIMITS OF CORNER BROOK PULP AND PAPER LIMITED Report prepared by Corner Brook Pulp & Paper Ltd to address the requirements of the Forest Stewardship Council, National Boreal Standard 2011 THE PRE-INDUSTRIAL CONDITION OF THE FOREST LIMITS OF CORNER BROOK PULP AND PAPER LIMITED Wayne A. Brown1 and E. Doyle Wells2 1 Wayne A. Brown, BScF, RPF, (Retired) Fibre Quality & Continuous Improvement Superintendent, Corner Brook Pulp & Paper Co. Ltd., Howley, NL 2 E. Doyle Wells, PhD, (Retired) Forest Ecologist, NRCan-CFS, St. John‟s, NL THE PRE-INDUSTRIAL CONDITION OF THE FOREST LIMITS OF CORNER BROOK PULP AND PAPER LIMITED EXECUTIVE SUMMARY The purpose of this report is to provide a knowledge base of the pre-industrial conditions (PIC) of the forest limits of Corner Brook Pulp and Paper Limited from which future forest management strategies can provide direction for the maintenance of forest ecosystems in perpetuity. For the purpose of this report, the pre-industrial period covers the time before the year 1890. Prior to that time, sawmilling was common and timber was removed for firewood and lumber. However, forest utilization occurred locally around coastal communities, and the impact on forest ecosystems was minimal. The completion of the cross-Island railway in the 1890s linked communities and provided access to the vast forest resources of the interior parts of the Island. Consequently, logging and sawmilling increased dramatically. However, the establishment, in 1909, of the first paper mill in central Newfoundland coincided with the decline of the lumber industry. This was followed in 1925 by the opening of the second paper mill in Corner Brook. -

Tap Water Quality for Public Water Supplies in Newfoundland and Labrador and Environment Physical Parameters and Major Ions
Department of Municipal Affairs Tap Water Quality for Public Water Supplies in Newfoundland and Labrador and Environment Physical Parameters and Major Ions Serviced Area(s) Source Name Sample Date Alkalinity Colour Conductivity Hardness pH TDS TSS Turbidity Boron Bromide Calcium Chloride Fluoride Potassium Sodium Sulphate Units mg/L TCU µS/cm mg/L mg/L mg/L NTU mg/L mg/L mg/L mg/L mg/L mg/L mg/L mg/L 15 6.5 - 8.5 500 1.0 5.0 250 1.5 200 500 Guidelines for Canadian Drinking Water Quality Aesthetic (A) or Contaminant (C) Parameter A A A C C A C A A Anchor Point Anchor Point Well Cove Brook Sep 17, 2019 106.00 10 243.0 120.00 8.28 158 0.60 LTD LTD 25.00 13 LTD LTD 7 1 Appleton Appleton (+Glenwood) Gander Lake (The Outflow) Aug 12, 2019 LTD 26 33.0 2.00 5.16 21 0.60 LTD LTD 1.00 7 LTD LTD LTD 1 Aquaforte Aquaforte Davies Pond Aug 21, 2019 12.00 14 107.0 LTD 7.79 70 0.40 LTD LTD LTD 24 LTD LTD 14 2 Arnold's Cove Arnold's Cove Steve's Pond (2 Intakes) Aug 21, 2019 12.00 10 61.0 5.00 7.21 40 0.60 LTD LTD 2.00 13 LTD LTD 9 1 Avondale Avondale Lee's Pond Sep 05, 2019 14.00 2 88.0 12.00 8.25 57 0.40 LTD LTD 5.00 15 LTD LTD 11 1 Baie Verte Baie Verte Southern Arm Pond Sep 26, 2019 LTD 19 34.0 5.00 5.05 22 0.20 LTD LTD 2.00 7 LTD LTD 2 1 Baine Harbour Baine Harbour Baine Harbour Pond Aug 29, 2019 8.00 83 38.0 5.00 7.33 25 0.40 LTD LTD 2.00 4 LTD LTD 4 2 Bartletts Harbour Bartletts Harbour Long Pond (same as Castors Sep 18, 2019 96.00 23 265.0 112.00 8.42 172 0.50 LTD LTD 25.00 22 LTD LTD 12 3 River North) Bauline Bauline #1 Brook Path Well Aug 15, -

Forestry in Newfoundland & Labrador
Chapter 9: Forestry in Newfoundland & Labrador INTRODUCTION TO FORESTS AND FOREST MANAGEMENT Figure 9.1: Sunset in the Boreal Forest. Did You Know? From space it is easy to see why Earth is called the blue planet. But a closer look Original Forests are reveals wisps of green. Those are our grasslands and forests. In fact, if you exclude sometimes thought of as Antarctica and Greenland where no trees grow, forests cover almost one third of the forests that existed long before Earth’s land—39 million square kilometres. significant human intervention. Information on original forests Even though they cover much less of the Earth’s surface than water, forests play a and today’s forests can be found major role in supporting life. They regulate climate, recycle moisture, stabilize soils, at: www.globalforestwatch.org. control runoff, recycle the air, provide food, and serve as natural habitats for almost two thirds of all Earth’s species. Scientists are just beginning to understand the important role forests play in the recycling of greenhouse gases and carbon—a process which can have a profound impact on climate change. Forests provide thousands of products that meet our everyday needs; wood fibre for paper, lumber, wood to heat our homes, and many commercial and traditional medicines. To ensure a sustainable source of these products, humans must take responsibility for protecting and preserving the forests and all their plants and animals that they support. Forestry in Newfoundland & Labrador 251 Increasing demands by humans for forest lands and products are depleting the Did You Know? world’s forests.