Migratory Patterns of Hilsa Shad in the Myanmar Ayeyarwady Delta Lessons for Fisheries Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Usg Humanitarian Assistance to Burma
USG HUMANITARIAN ASSISTANCE TO BURMA RANGOON CITY AREA AFFECTED AREAS Affected Townships (as reported by the Government of Burma) American Red Cross aI SOURCE: MIMU ASEAN B Implementing NGO aD BAGO DIVISION IOM B Kyangin OCHA B (WEST) UNHCR I UNICEF DG JF Myanaung WFP E Seikgyikanaunglo WHO D UNICEF a WFP Ingapu DOD E RAKHINE b AYEYARWADY Dala STATE DIVISION UNICEF a Henzada WC AC INFORMA Lemyethna IC TI Hinthada PH O A N Rangoon R U G N O I T E G AYEYARWADY DIVISION ACF a U Zalun S A Taikkyi A D ID F MENTOR CARE a /DCHA/O D SC a Bago Yegyi Kyonpyaw Danubyu Hlegu Pathein Thabaung Maubin Twantay SC RANGOON a CWS/IDE AC CWS/IDE AC Hmawbi See Inset WC AC Htantabin Kyaunggon DIVISION Myaungmya Kyaiklat Nyaungdon Kayan Pathein Einme Rangoon SC/US JCa CWS/IDE AC Mayangone ! Pathein WC AC Î (Yangon) Thongwa Thanlyin Mawlamyinegyun Maubin Kyauktan Kangyidaunt Twantay CWS/IDE AC Myaungmya Wakema CWS/IDE Kyauktan AC PACT CIJ Myaungmya Kawhmu SC a Ngapudaw Kyaiklat Mawlamyinegyun Kungyangon UNDP/PACT C Kungyangon Mawlamyinegyun UNICEF Bogale Pyapon CARE a a Kawhmu Dedaye CWS/IDE AC Set San Pyapon Ngapudaw Labutta CWS/IDE AC UNICEF a CARE a IRC JEDa UNICEF a WC Set San AC SC a Ngapudaw Labutta Bogale KEY SC/US JCa USAID/OFDA USAID/FFP DOD Pyinkhayine Island Bogale A Agriculture and Food Security SC JC a Air Transport ACTED AC b Coordination and Information Management Labutta ACF a Pyapon B Economy and Market Systems CARE C !Thimphu ACTED a CARE Î AC a Emergency Food Assistance ADRA CWS/IDE AC CWS/IDE aIJ AC Emergency Relief Supplies Dhaka IOM a Î! CWS/IDE AC a UNICEF a D Health BURMA MERLIN PACT CJI DJ E Logistics PACT ICJ SC a Dedaye Vientiane F Nutrition Î! UNDP/PACT Rangoon SC C ! a Î ACTED AC G Protection UNDP/PACT C UNICEF a Bangkok CARE a IShelter and Settlements Î! UNICEF a WC AC J Water, Sanitation, and Hygiene WC WV GCJI AC 12/19/08 The boundaries and names used on this map do not imply official endorsement or acceptance by the U.S. -

D E D a Y E K Y a I K L a T B O G a L E Pyapon Mawlamyinegyun
95°30’0"E 95°40’0"E 95°50’0"E TAUNGBOGON NGA-EINDAN KWINGYAUNG KALAGYI KALAUNGBON DAUNGGYI MIGYAUNGAING YWA-BIT YWAHAUNG MAYAN KYUNGYA MAYAN TA M AN G YI KALAGYIWA YOKSAING GYOWA GONDANGALE KUNBINGYAUNG MALAGON NPOPON YWATHIT KYONSOK ONGYI TA M U T TALOKSEIK KUNGYANGON TAUNGALE MINHLAZU MAYAN AMAWCHOK KYAUKYEZU KYAGON THEGON TA I N G KWI HTEINGAING NGE-EINZU KYONKYAIK KYONBE LE-EINZU AINGBON TEIKPWIN TANYINGON Mawlamyinegyun TA M O N KYONTA MEZALIGAN HPONYOZEIKASU KYIBINZU SHANGWIN NYAUNGGYAUNG Kyaiklat TA M AWG Y I LINDAING KANZU TA M AN MINHLA-ASU HNGETTAW TETTEZU THEINGONGYI HKANAUNG KYAGAYET YWATHIT-ASHE TA M AW- ATE T CHAUKEINDAN MAYITKA-KWIN KUNBIN THALEIK KANZU MA-UBIN KULAN-MYAUK THAYAGON HTALUNZU INDU DABAYIN MINGAN KULAN-TAUNG NYINAUNG NANGYAUNG MYINGAGON HKANAUNG-ASHE AKHA KULAN-MYAUK LAMUGYI SHANZU AGEGYI PETALA BOGALE TEINBIN BONTHALEIK DANIZU KOTHETSHE-ASU ASIGALE TA M AN KWI N TAW H KA M AN KYONDU KYONTHUT-ASHE HSATTHABUGON 16°20’0"N KYUNGYA THANLAIK PETTETAUNG 16°20’0"N THE-EIN KAYINZU HMAWBI HMAWAING TAW H L A WEGYI HAINGSI YWATHIT THAKAN CHAUNGDWIN TA M AN G YI GWEDAAUKKON LETPYAUNGBAING THEGONGALE YWADANSHE THITTOGYAUNG PAYA GY IGO N POYAUNG THE-EINGYAUNGZU THAYAGON KAYINZU SAYAYO-ASU AKYI MAYANGWA MEZALIGYAUNG ONBIN PA-AUNGGYI PANGADAT SHANGWIN KALAGYICHAUNG TEBINZEIK THAKAN DANIBAT KYONKU KWIN KHAMAPO UDO KONDAN YEGYAW-YWA POSHWELON-ASU MANGEGALE KANZU KYAUNGZU DedayeTA N YI PAYA GYAUNG MAGYIDAN DANIPAT EINYAGYI KUNTHICHAUNGWA KYONPA TA M AN NEYAUNGGON KYONTHUT-MYAUK APYAUNG SITKON KOTAIKKYI-ASU PAUK PA NBY UZU MYINGAGON -
B O G a L E Mawlamyinegyun Wakema Labutta M Y a U N G M Y a N
94°20’0"E 94°30’0"E 94°40’0"E 94°50’0"E 95°0’0"E 95°10’0"E CHAUNGAUK KYETTHUNGYAUNG DAYIN-GAUK PEINNEGYAUNG YEGYAN KONGALE TUMYAUNG ONBINZEIKPONSOGYI BYAINGDAUNG YWATHIT NYAUNGLAN UDOCHAUNG MEZALI-UDO YEGYAWGYI KYAUKKWE KYAGWIN HM ANGU KWINBAT KYAUKPON POBYE KANGYAUNG THONGWA KYAWNU-UDO HGETKYIDAN SHWEKA YWATHITGALE NGAYOKTHI THAYETKON LABUTPYE SHWEZAN THEGYAUNG SEIKKYI YWATHITGALE KYAUKPYAGALE PAWDAW MU YWATHIT MEZALI MANKALA THAYETCHAUNG LEGWA TIKOGAING KYAUKKWE BEBAUK KYONLATA KYUNTHIT WakemaNYAUNGGYAUNG KANGON LE-EINDAN KALAMATAUNG TA U KS H A PACHAUNG KYEINNI SABYUZU SHANZU THALIGAGON HM ANGU SABYUZU TALAPHIKYUN HTANNYETCHAUNG ALEGYAUNG AUNGHLAING 16°20’0"N KINMONZEIK NONKYUN-AUKSU 16°20’0"N MAUNG-BI KYEINGONGYI THAYEGYAUNG BUDINGYAUNG ZAYATCHAUNGBYA PEIKTA DUNWAING PEINGYAUNG YAKAINGGON THINGANBYU YAMALNW KYONLATA-AWA MYINDALIN LEIKABO KYIGYAUNG POLAUNG KOKKO KANGYAUNG KUNGWIN THAINGGYAUNG MyaungmyaLEBYAUK KYONLAMU MYATTHA-UDO POYANGON KANAZOGYAUNG NYAUNGBINTHA KWINGYAUNG MINGON MYITKYO SETKON NGADA NTAY TEBIN MOGAUNG MAYANGON EINMAGON KYUDAW THAUNGBON KYONLATA BYAUNGBYAN PO-SHWE HLAW MEZALI THEGYAUNG MYAHPUGYAUNG PYINMAGYAUNG-WA KHAYEGON KYAGAN KYAGAN PYAKEIK SE-EINZU KYAGYAUNG NGAYANGAUNGDO KYUNGALE MWEHAUK THEGON SAMALAUK CHAUNGBYA MABE POTILUT CHAUNGBYA KATHABAUNG AWABEIK CHAUNGGWEGYI AUNGHLAING NAT-HMU KYUTKON CHAUNGGWE KYAUKGYI ALEYWA THINDAWGYI PEIKTAGYI SHAUKCHAUNG YWATHIT MAYANGON KYAUKPYAKWIN BAWINSU KYAUKPYA LEIKPOK KYUNGYAUNG THAHTEGON THAMA AH-KA YWATHIT YEGYOGON HLAINGBON SANGYIGON THAYETPINGWIN KABALU NY EINU DIPAYON -

The Provision of Public Goods and Services in Urban Areas in Myanmar: Planning and Budgeting by Development Affairs Organizations and Departments
The Provision of Public Goods and Services in Urban Areas in Myanmar: Planning and Budgeting by Development Affairs Organizations and Departments Michael Winter and Mya Nandar Thin December 2016 Acknowledgements The authors thank the many Development Affairs Organization (DAO) officials in Shan, Mon and Kayin States and in Ayeyarwady and Tanintharyi Regions who discussed their work and generously provided access to DAO documentation. The authors would also like to thank members of Township Development Affairs Committees (TDACs) who contributed to the production of this report. In addition, the authors thank the staff of The Asia Foundation and Renaissance Institute for providing invaluable logistical and administrative support. About the Authors Michael Winter, the lead author of the report, over the last twenty years, has worked as a consultant on local government and local development issues in Asia and Africa. His main clients have included UNCDF, UNDP, the World Bank, the Asian Development Bank, SDC, and the UK’s Department for International Development (DFID). Mya Nandar Thin is a Program Associate at Renaissance Institute and provides support in the planning and implementation of research and advocacy activities lead by the Public Financial Management Reform team. About The Asia Foundation and Renaissance Institute The Asia Foundation is a nonprofit international development organization committed to improving lives across a dynamic and developing Asia. Informed by six decades of experience and deep local expertise, our programs address critical issues affecting Asia in the 21st century—governance and law, economic development, women’s empowerment, environment, and regional cooperation. In addition, our Books for Asia and professional exchanges are among the ways we encourage Asia’s continued development as a peaceful, just, and thriving region of the world. -

UNDP Myanmar Responds to Cyclone Nargis
UNDP Myanmar responds MATTERS OF FACT to Cyclone Nargis • 40 UNDP and its implementing partner NGO PACT offices in the Ayeyarwady Delta UNDP moved into action within 24 hours after Cyclone • 23 field teams active in the worst affected areas Nargis hit Myanmar on 2-3 May. The storm carved a • 500 national staff and project personnel working in the path of destruction that left 133,653 dead or missing delta and being mobilized for Cyclone Nargis response and 2.4 million severely affected by the crisis. operations • 5 UNDP offices functioning as ‘base camp’ for UN The cyclone’s 120-mile per hour winds and resulting organizations and international NGOs delivering to, and storm surge were particularly devastating in the working in Bogale, Mawlamyinegyun, Labutta, Ayeyarwady Delta, where entire villages were flattened. Ngapudaw and Kyaiklat • 2.4 million people severely affected by Cyclone UNDP is the only UN organization with field offices Nargis across Myanmar located in the region, which, prior to the cyclone was • 1.4 million people affected in the Ayeyarwady delta, home to seven million people. UNDP staff and families the hardest hit region of Myanmar experienced the natural disaster first-hand, as did those • 43,241 estimated total beneficiaries from UNDP coordinated relief efforts as of 21 May who work for partner non-governmental organization (NGO) PACT, which lost five of its project personnel. Supporting relief efforts: UNDP sent rotating teams UNDP has 40 functioning field offices in the delta and of national staff to work with and relieve its field staff in current field staff strength of more than 500 five of the affected townships – Bogale, experienced national staff and project personnel, Mawlamyinegyun, Labutta, Ngapudaw and Kyaiklat – including those of PACT. -

Cyclone Nargis
Emergency appeal n° MDRMM002 Myanmar: GLIDE n° TC-2008-000057-MMR Operations update n° 11 23 May 2008 Cyclone Nargis Period covered by this Update: one week since revised emergency appeal launched Revised Emergency Appeal launched 16 May 2008: CHF 52,857,809 (USD 50.8 million or EUR 32.7 million) to assist 100,000 families for three years; <click here to view the attached Emergency Appeal Budget> The revised plan of action covers the provision of life- saving assistance and short-term relief (for up to six months) as well as medium and longer term recovery needs. It aims to support a significant scaling up in the Building trust: MRCS volunteers let humanitarian response of Myanmar Red Cross Society families speak for themselves in terms of (MRCS) as well as the wider Red Cross Red Crescent what they need. Movement. The appeal seeks to do this in a way that: is sensitive to the national society’s capacity; builds on MRCS’s long term strengths; and takes account of the operational challenges that exist. In light of all this, partners are requested to continue their excellent support and understanding to the Cyclone Nargis appeal. <Click here to link to the donor response list> <click here to link to contact details > Appeal history: • 16 May 2008: A revised emergency appeal launched for CHF 52,857,809 (USD 50.8 million or EUR 32.7 million) to assist 100,000 families for three years • 6 May 2008: A preliminary emergency appeal launched for CHF 6,290,909 (USD 5.9 million or EUR 3.86 million) for six months to assist 30,000 families. -

Hazard Profile of Myanmar: an Introduction 1.1
Table of Contents Table of Contents ............................................................................................................ I List of Figures ................................................................................................................ III List of Tables ................................................................................................................. IV Acronyms and Abbreviations ......................................................................................... V 1. Hazard Profile of Myanmar: An Introduction 1.1. Background ...................................................................................................................... 1 1.2. Myanmar Overview ......................................................................................................... 2 1.3. Development of Hazard Profile of Myanmar : Process ................................................... 2 1.4. Objectives and scope ....................................................................................................... 3 1.5. Structure of ‘Hazard Profile of Myanmar’ Report ........................................................... 3 1.6. Limitations ....................................................................................................................... 4 2. Cyclones 2.1. Causes and Characteristics of Cyclones in the Bay of Bengal .......................................... 5 2.2. Frequency and Impact .................................................................................................... -

Myanmar: Tropical Cyclone Nargis (As of 16 May 2008)
Minbu Myanmar: Tropical CycloneTaungdwingyi Nargis (as of 16 May 2008) Cyclone Nargis Track • Population in disaster-declared GMT +6:30 areas: approx. 24 million CHINA • In Yangon: approx. 6 million INDIA • UN est.: 1.6 - 2.5 million people SITUATION severely affected • 78,000 dead MYANMAR 4 May • Cyclone Nargis struck Myanmar • 56,000 missing LAO P.D.R. on 2 and 3 May 2008, sweeping • 1,430 injured 3 May Toungoo through the Ayeyarwady Delta • >550,000 in temporary shelters 2 May THAILAND region and the country’s largest • 20% of children in affected areas city, Yangon. suffering from diarrhoea CAMBODIA • Food, shelter, medical supplies • 3,000 schools destroyed/ damages MYANMAR ANDAMAN and water are critical needs affecting 500,000 children SEA Pyu Gulf of • growing risk of an outbreak of • WFP dispatched >1,195 tons of Myanaung Thailand food and distributed 571 tons of infectious disease food to 159,900 beneficiaries Bago Logistics • 27 flights with relief items landed to date; 32 more • Before the cyclone, Travelling times to reach population centres in planned or confirmed to arrive 30% chronic and 9% some affected areas (in hours): Nyaunglebin accute malnutrition Yangon - Kyaiklat 3.0 by road • 79 international staff and 1,574 Bay of • Nutrition assessment Yangon - Bogale 4.5 by road national staff in Myanmar as of completed; analysis Yangon - Pyapon 3.5 by road 30 April 2008 Bengal in process Pyapon - Mawlamyinegyun 1.5 by river (motorised boat) Source: World Vision (as of 14 May 2008) I r LINKS a Thanatpin • WFP set up two Ayeyarwady -

Probable Flood Inundated Area in Lemyethna, Yegyi and Thabaung Townships (As of 30 August 2020, 06:15 AM)
Probable Flood Inundated Area in Lemyethna, Yegyi and Thabaung Townships (as of 30 August 2020, 06:15 AM) !. Kyaung Kwin Taw Ywar Kyi Gyee Yae Hpyu Zee Taw Lay Ein Tan Kone Su Let U Su 95°0'E 95°15'E Kyat Pyay (Ah Lel Su) Ka Nyin Taing Bi Tha Lun Ma Thea Kone M inb u Zee Pin Kwin Kyar Kaik Ka Nyin Ngu Ngape M agway Nay Pyi Taw Kyee Kone Khat Tu Gyan Kone Pinlaung Taung Poet Pi Aung Baw Su Moe Kaung Su Taungd wingyi Taung Kone Kyar Kaik Kun Chan Kone Me Za Li Kwin Sin Thay (North) (!^_ Loikaw Nyaung Chay Htauk Kyun Te Sit Kone M inhla Pyinm ana Bant Bway Kone Hman Tan Myo Ma Oke Pon Za Yat Kwin Kyu Taw Pekon !.Loikaw Ngar Poke Kwin Sar Yay Kwin Za Yit Yoe Ann Sinb aungwe Ma Gyi Kone Bo Yon Su Lem ye thna Dem oso Kone Gyi Lewe Taung Ka Lay Saw Pyar Yoe Gyi Tha Pyu Yoe Sein Baung Sein Baung Ah Kei Saw Pyar Khon Gyi Kyon Pi Khat Cho Kone Thaye t Htone Bo Ngar Khone Ma Chaung Doke Yaik Kyaukp yu M ind on Tha Khut Chaung Kyar Ni Taung Pay Kone Kyoet Kone Aunglan Y e d ashe Hpruso Nat Hta Min Kone Daunt Gyi Lel Di Su Pan Be Kone Ramree San Gyi Nyaung Waing Kyar Inn Bawlake Wea Daunt / Kamm a Thand aunggyi Yae Thoe Oke Aing Ta Loke Kone Kyaung Kone Toungup Taungoo Lem ye thna Sein Taung Thein Kone Paukkhaung Tha Khwar Kone Kyaung Kone Ywar Thit Kone Pyay Ma Yan Kone Daunt Gyi Hpasawng Chin Lel Sein Taung Moe Goke War Yon Chaung Ah Nyar Su Township U Yin Kone Lel U Su Kyat Kone Ka Nyin Chaung Oktwin Myin Kyoe Chaung Kywe Mee Swea Yae Thoe Chaung Hlaw Ka Htar Hlaw Ka Htar Kha Paung Kwin Gway Cho M e se Ngar Me Aing Padaung Leik Khone Paungd e Thin -
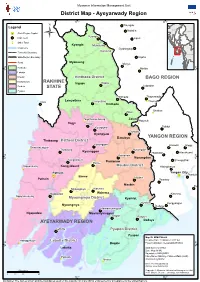
Ayeyarwady Region
Myanmar Information Management Unit District Map - Ayeyarwady Region 95° E 96° E Paungde Legend INDIA Nattalin CHINA .! State/Region Capital Kyangin Main Town Ü Zigon !( Other Town Kyangin Myanaung Coast Line !( Gyobingauk Kanaung Township Boundary THAILAND State/Region Boundary Okpho Road Myanaung Monyo N Kyeintali N ° Hinthada !( ° 8 Minhla 8 1 1 Labutta Maubin Hinthada District BAGO REGION Myaungmya RAKHINE Ingapu Ingapu Pathein STATE Letpadan Pyapon Hinthada Thayarwady Gwa Lemyethna Lemyethna !( Thonse Hinthada Okekan Zalun !( Ngathaingchaung Zalun !( Ahpyauk !( Yegyi Yegyi Kyonpyaw Taikkyi Danubyu Kyonpyaw Danubyu YANGON REGION Thabaung Pathein District Kyaunggon Hmawbi Hlegu Shwethaungyan !( Thabaung Nyaungdon Kyaunggon Htantabin Htaukkyant N N ° Pantanaw ° 7 7 1 Nyaungdon 1 Kangyidaunt Pantanaw Shwepyithar Einme Ngwesaung Kangyidaunt Maubin District !( Hlaingtharya Pathein Yangon City .! .! Thanlyin Einme Maubin Pathein Twantay Maubin Kyauktan Myaungmya Wakema Ngapudaw Wakema Kawhmu Ngayokekaung !( Myaungmya District Kyaiklat Kyaiklat Kungyangon Myaungmya Dedaye Mawlamyinegyun Ngapudaw Mawlamyinegyun Bogale Pyapon AYEYARWADY REGION Dedaye Labutta Pyapon District Pyapon Map ID: MIMU764v04 Hainggyikyun Creation Date: 23 October 2017.A4 N Labutta District N !( ° Bogale Projection/Datum: Geographic/WGS84 ° 6 6 1 1 Labutta Data Sources: MIMU Base Map: MIMU Boundaries: MIMU/WFP Pyinsalu Place Name: Ministry of Home Affairs (GAD) !( Ahmar translated by MIMU !( Email: [email protected] Website: www.themimu.info Kilometers Copyright © Myanmar Information Management Unit 2017. May be used free of charge with attribution. 0 10 20 40 95° E 96° E Disclaimer: The names shown and the boundaries used on this map do not imply official endorsement or acceptance by the United Nations.. -

Mimu939v01 29Nov12 SDC S
Myanmar Information Management Unit SDC-HA School/Storm Shelter Project Bogale, Labutta, Mawlamyinegyun and Pyapon Townships, Ayeyarwady Region 94°30'E 94°40'E 94°50'E Myaungmya 95°E 95°10'E Wakema 95°20'E 95°30'E 95°40'E 95°50'E Twantay Einme Maubin BHUTAN INDIA Kachin CHINA Ngapudaw Ü Sagaing Chin Shan Mandalay Magway LAOS Kyaiklat Kayah Rakhine Bago 16°30'N Myaungmya 16°30'N Yangon Kawhmu Wakema Ayeyarwady Kayin THAILAND Mon Ngapudaw Tanintharyi Kyaiklat Tone Hle Ywa Ma (Myo Ma Ward (8)) ! Mawlamyinegyun Bar Thar Kone (Bar Thar Kone) ! ! Ywar Thit Mawlamyinegyun (Min Bu Su) 16°20'N Thar Li Kar Kone Hta Nyet Chaung Hpa Yar Gyi Kone/Hpa Yar Kone 16°20'N (Thar Li Kar Kone) (Me Za Li U To) (Bant Bway Su) ! ! ! Dedaye Bogale Hpoe Shwe Lone (Haing Si) ! Pyapon Myat Thar Ywar Ma Yae Hpyu Kan (Myat Thar Ywar Ma) (Ka Ka Yan) ! ! Thaung Hpone Bay Pauk (Shauk Chaung) Da Ni Chaung (Shaw Chaung) ! (Kyet Shar) ! ! Yae Ka Myin (Ah Lel Yae Kyaw) ! Pyapon 16°10'N 16°10'N Labutta Ah Lan Hpa Lut (Ah Lan Hpa Lut) ! Ka Zaung Ma/ Ka Yaung Ma (Nga Pyay Ma) ! Bogale Ah Htet Hpoe Nyo (Nga Pyay Ma) Let Pan ! (Let Pan Pin) ! Kyon Ka Dun (Kyon Ka Dun) Set San Hnget Ka Lay Kwin East 2 ! (Set San) (Zin Baung) ! ! ! Aung Chan Thar ! (Set San) Hnget Ka Lay Kwin East 1 (Zin Baung) War Chaung (Day Da Lu) ! Tar Pun Thea Chaung Lay Gwa Saing (Day Da Lu ) (Daunt Gyi) (Daunt Gyi) ! Ah Si Gyi ! ! (Set San) Oke Twin 16°N ! (Day Da Lu ) 16°N ! Boe Su Chaung Hpa Yar Gyi Kone (Day Da Lu ) (Myo Kone ) ! ! !Hpo Khway Yoe (Myo Kone ) Shwe Htee (Day Da Lu ) ! Labutta Kan Su (Myo -

Socioeconomic Characteristics of Hilsa Fishers in the Ayeyarwady Delta, Myanmar Opportunities and Challenges
A position Paper Prepared for the New Perspectives on Climate Resilient Drylands Development Project Socioeconomic characteristics of hilsa fishers in the Ayeyarwady Delta, Myanmar Opportunities and challenges Wae Win Khaing, Michael Akester, Eugenia Merayo Garcia, Annabelle Bladon and Essam Yassin Mohammed Country Report Fisheries; Poverty Keywords: December 2018 Sustainable fisheries; ocean; poverty reduction; livelihoods; communities About the authors Wae Win Khaing, Network Activities Group; Michael Akester, WorldFish Myanmar, Eugenia Merayo Garcia, International Institute for Environment and Development; Annabelle Bladon, International Institute for Environment and Development; Essam Yassin Mohammed, International Institute for Environment and Development. Corresponding author email [email protected] Produced by IIED’s Shaping Sustainable Markets group IIED's Shaping Sustainable Markets group works to make sure that local and global markets are fair and can help poor people and nature thrive. Our research focuses on the mechanisms, structures and policies that lead to sustainable and inclusive economies. Our strength is in finding locally appropriate solutions to complex global and national problems. Acknowledgements We would like to thank all participants of the socioeconomic survey that formed the basis of this report – particularly the Ayeyarwady Delta fisherfolk. We thank the Ayeyarwady Region Department of Fisheries, township Department of Fisheries offices, and the Myanmar Fisheries Federation for their support. We also recognise the contributions of WorldFish, the FISH CGIAR Research program, and Bobby Maung and Myo Zaw Aung from the Network Activities Group. Published by IIED, December 2018 Wae Win Khaing, Michael Akester, Eugenia Merayo Garcia, Annabelle Bladon and Essam Yassin Mohammed (editor) (2018) Socioeconomic characteristics of hilsa fishers in the Ayeyarwady Delta, Myanmar: Opportunities and challenges.