Existence of Non-Agglutinating Aeromonas Salmonicida Subsp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Movie and Art Country: Myanmar
JENESYS2019 ASEAN Inbound Program 4th Batch Program Report Theme: Movie and Art Country: Myanmar 1. Program Overview 11 youths from Myanmar who had interests in movie and art visited Japan for a period of 9 days from October 8 to 16, 2019 as part of JENESYS 2019 under the theme of “Movie and Art”. The delegations attended a theme-related lecture in Tokyo and observed museums, university, and art galleries to deepen the knowledge of Japanese art. Afterwards, they visited Yamagata prefecture and participated in the Yamagata International Documentary Film Festival as well as had some opportunities to discuss the expressions of movie and art with stakeholders of the festival. It promoted interactions between Japan and Myanmar and deepened the friendly relationship between two countries. During the program, the participants showed strong interests in Japanese movie productions and unique cultures, as well as shared their discoveries and experiences in Japan through Social Media. At a reporting session before leaving Japan, the group presented an action plan (activity plans after returning home) to convey their experience while visiting Japan. 【Participating Countries and Numbers of Participants】 11 persons from Myanmar 【Prefectures Visited】 Tokyo, Yamagata Prefecture 2. Program Schedule October 8th (Tue) 【Arrival】 【Courtesy Call】Embassy of the Republic of the Union of Myanmar 【Orientation】 【Theme-related Observation】Printing Museum October 9th (Wed) 【Company Visit】NPO Kogane-cho Area Management Center 【School Visit】Tokyo University of the Arts 【Theme-related Observation】Bank ART Home & SILK 1 October 10th (Thu) 【Theme-related Observation】Tokyo National Museum 【Theme-related Observation】Akihabara - Move from Tokyo to Yamagata October 11th (Fri) 【Theme-related Observation】Yamagata International Documentary Film Festival 【Company Visit】TV-U Yamagata Inc. -

Local Dishes Loved by the Nation
Sapporo 1 Hakodate 2 Japan 5 3 Niigata 6 4 Kanazawa 15 7 Sendai Kyoto 17 16 Kobe 10 9 18 20 31 11 8 ocal dishes Hiroshima 32 21 33 28 26 19 13 Fukuoka 34 25 12 35 23 22 14 40 37 27 24 29 Tokyo loved by 41 38 36 Nagoya 42 44 39 30 Shizuoka Yokohama 43 45 Osaka Nagasaki 46 Kochi the nation Kumamoto ■ Hokkaido ■ Tohoku Kagoshima L ■ Kanto ■ Chubu ■ Kansai 47 ■ Chugoku ■ Shikoku Naha ■ Kyushu ■ Okinawa 1 Hokkaido 17 Ishikawa Prefecture 33 Okayama Prefecture 2 Aomori Prefecture 18 Fukui Prefecture 34 Hiroshima Prefecture 3 Iwate Prefecture 19 Yamanashi Prefecture 35 Yamaguchi Prefecture 4 Miyagi Prefecture 20 Nagano Prefecture 36 Tokushima Prefecture 5 Akita Prefecture 21 Gifu Prefecture 37 Kagawa Prefecture 6 Yamagata Prefecture 22 Shizuoka Prefecture 38 Ehime Prefecture 7 Fukushima Prefecture 23 Aichi Prefecture 39 Kochi Prefecture 8 Ibaraki Prefecture 24 Mie Prefecture 40 Fukuoka Prefecture 9 Tochigi Prefecture 25 Shiga Prefecture 41 Saga Prefecture 10 Gunma Prefecture 26 Kyoto Prefecture 42 Nagasaki Prefecture 11 Saitama Prefecture 27 Osaka Prefecture 43 Kumamoto Prefecture 12 Chiba Prefecture 28 Hyogo Prefecture 44 Oita Prefecture 13 Tokyo 29 Nara Prefecture 45 Miyazaki Prefecture 14 Kanagawa Prefecture 30 Wakayama Prefecture 46 Kagoshima Prefecture 15 Niigata Prefecture 31 Tottori Prefecture 47 Okinawa Prefecture 16 Toyama Prefecture 32 Shimane Prefecture Local dishes loved by the nation Hokkaido Map No.1 Northern delights Iwate Map No.3 Cool noodles Hokkaido Rice bowl with Tohoku Uni-ikura-don sea urchin and Morioka Reimen Chilled noodles -

Studies on the Genesis of Metallic Mineral Deposits of Shin Jo and Yamagata Basins, Northeastern Japan (I)
岩 石鉱 物鉱 床学 会誌 60巻4号, 1968年 STUDIES ON THE GENESIS OF METALLIC MINERAL DEPOSITS OF SHIN JO AND YAMAGATA BASINS, NORTHEASTERN JAPAN (I) NORITSUGU OIZUMI Mining Division Prefectuval Governient of Yamagata The present structural set up of the Shinjo and Yamagata basins which are situated within the Inner Region, Northeastern Japan, is a result of repeated uplift and submergence coupled with igneous activity and sedimentation during the early stage of the Neogene. Those structur es formed as a result of uplift of the basement are the major faults and fissures parallel to the N-S trend of the present axis of the Basement Rise and the subordinate faults and fissures trending E-W and perpen dicular to that axis Those formed as a result of folding of the sedimentary rocks are the NNW-SSE trending structures of northern Shinjo Basin, the N-S trending structures of southern Shinjo Basin, the N-S trending structures of northern Yamagata Basin, and the NNE-SSW trending structures of southern Yamagata Basin. These structures include the fold axes and the faults and fissures parallel to them. In addition to these structures produced by folding of the sedimentary rocks, are the subordinate faults and fissures trending WNW and NE-SW to ENE. The crushed zones within these fractures were produced by an E-W lateral compression, which also produced exceedingly abundant E-W trending tension fractures, The NNE-SSE trending fissures adhere closely to these fractures caused by lateral compression. The individual ore deposits present within the two basins amount to a total of more than 300 ore deposits. -

A Retrospective Study of Yamagata Prefecture, Japan, 1984–2014
Epidemiol. Infect. (2017), 145, 462–470. © Cambridge University Press 2016 doi:10.1017/S0950268816002430 Meteorological factors affecting scrub typhus occurrence: a retrospective study of Yamagata Prefecture, Japan, 1984–2014 1 1 2 3 1 J. SETO *, Y. SUZUKI ,R.NAKAO,K.OTANI,K.YAHAGI AND K. MIZUTA1 1 Department of Microbiology, Yamagata Prefectural Institute of Public Health, Yamagata City, Yamagata, Japan 2 Laboratory of Parasitology, Department of Disease Control, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Hokkaido, Japan 3 Department of Public Health, Yamagata University Graduate School of Medicine, Yamagata, Japan Received 10 May 2016; Final revision 31 August 2016; Accepted 28 September 2016; first published online 28 October 2016 SUMMARY Climate change, by its influence on the ecology of vectors might affect the occurrence of vector- borne diseases. This study examines the effects of meteorological factors in Japan on the occurrence of scrub typhus, a mite-borne zoonosis caused by Orientia tsutsugamushi. Using negative binomial regression, we analysed the relationships between meteorological factors (including temperature, rainfall, snowfall) and spring–early summer cases of scrub typhus in Yamagata Prefecture, Japan, during 1984–2014. The average temperature in July and August of the previous year, cumulative rainfall in September of the previous year, snowfall throughout the winter, and maximum depth of snow cover in January and February were positively correlated with the number of scrub typhus cases. By contrast, cumulative rainfall in July of the previous year showed a negative relationship to the number of cases. These associations can be explained by the life-cycle of Leptotrombidium pallidum, a predominant vector of spring–early summer cases of scrub typhus in northern Japan. -

What Is Dewa Sanzan? the Spiritual Awe-Inspiring Mountains in the Tohoku Area, Embracing Peopleʼs Prayers… from the Heian Period, Mt.Gassan, Mt.Yudono and Mt
The ancient road of Dewa Rokujurigoegoe Kaido Visit the 1200 year old ancient route! Sea of Japan Yamagata Prefecture Tsuruoka City Rokujurigoe Kaido Nishikawa Town Asahi Tourism Bureau 60-ri Goe Kaido Tsuruoka City, Yamagata Prefecture The Ancient Road “Rokujuri-goe Kaido” Over 1200 years, this road has preserved traces of historical events “Rokujuri-goe Kaido,” an ancient road connecting the Shonai plain and the inland area is said to have opened about 1200 years ago. This road was the only road between Shonai and the inland area. It was a precipitous mountain road from Tsuruoka city to Yamagata city passing over Matsune, Juo-toge, Oami, Sainokami-toge, Tamugimata and Oguki-toge, then going through Shizu, Hondoji and Sagae. It is said to have existed already in ancient times, but it is not clear when this road was opened. The oldest theory says that this road was opened as a governmental road connecting the Dewa Kokufu government which was located in Fujishima town (now Tsuruoka city) and the county offices of the Mogami and Okitama areas. But there are many other theories as well. In the Muromachi and Edo periods, which were a time of prosperity for mountain worship, it became a lively road with pilgrims not only from the local area,but also from the Tohoku Part of a list of famous places in Shonai second district during the latter half of the Edo period. and Kanto areas heading to Mt. Yudono as “Oyama mairi” (mountain pilgrimage) custom was (Stored at the Native district museum of Tsuruoka city) booming. -

Activities in Japan 1 Activities in Japan
Chapter 3 Activities in Japan 1 Activities in Japan (1) Schedule Date Time Program October 27 <National Leaders (NLs), Participating Youths (PYs) and host family representatives Tuesday from ASEAN member countries> Arrival at Narita International Airport 6:45 Myanmar (NH-814) 7:15 Malaysia, Brunei Darussalam (MH-088) 7:35 Lao P.D.R., Cambodia (TG-642) 8:00 Host family representatives from Vietnam (VN-300) 8:50 Indonesia (GA-874) *arrival at Haneda airport Transfer to the Cabinet Office for orientation Move to Hotel New Otani Tokyo 15:00 Philippines (NH-820) 15:05 Vietnam (VN-384) *arrival at Haneda airport 16:05 Singapore (JL-712) 17:30 Thailand (JL-032)*arrival at Haneda airport Transfer to Hotel New Otani Tokyo and orientation at the hotel Stay at Hotel New Otani Tokyo <Japanese PYs> Pre-departure training Stay at National Olympics Memorial Youth Center October 28 <Japanese PYs> Wednesday 8:15 Move to Hotel New Otani Tokyo <NLs, PYs and host family representatives> 9:00-11:00 Orientation (“Ho-oh”, Hotel New Otani Tokyo) • Speech by Mr. Hideki Uemura, Administrator • Introduction of NLs and PYs • Introduction of host family representatives • Introduction of Administrative staff members • Explanation of the country program in Japan • Speech by Ms. Tomoko Okawara, Chairperson of Japan-ASEAN Youth Leaders Summit (YLS) Organizing Committee • Solidarity Group (SG) meeting <Host family representatives> 11:15-11:45 Courtesy call on Mr. Takahiko Yasuda, Director General for International Youth Exchange, Cabinet Office (“Tsubaki”, Hotel New Otani Tokyo) • Speech by Mr. Takahiko Yasuda, Director General for International Youth Exchange, Cabinet Office • Presentation of certificate and gift • Photo session 30 Chapter 3 Activities in Japan Date Time Program October 28 <NLs, PYs and host family representatives> Wednesday 12:00-12:30 Inauguration Ceremony (“Ho-oh”, Hotel New Otani Tokyo) • Moment of silence for the victims of the bus accident in Brunei Darussalam in 2001 • Speech by Mr. -
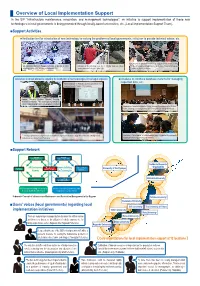
Overview of Local Implementation Support
Overview of Local Implementation Support In the SIP "Infrastructure maintenance, renovation, and management technologies", an initiative to support implementation of these new technologies in local governments is being promoted through locally-based universities, etc. (Local Implementation Support Team). ■Support Activities ●Verification test for introduction of new technology for solving the problems of local governments, initiatives to provide technical advice, etc. Verification of application of advanced magnetic non-destructive testing Gifu University (Professor Rokugo) Verification of the use of robotic Verification of the performance on site of a bridge inspection robotic device for evaluation of degradation of the bottom of columns for signage technology on site (Gifu University, Kakamigahara City) camera (Nagasaki Prefecture, Saikai City) (Osaka city, Osaka Prefecture) ●Initiatives to spread information regarding the introduction of new technologies, for training of engineers ●Initiatives to introduce database systems for managing inspection data, etc. Public symposium by Hokkaido University and officials from local governments (Sapporo City, Hokkaido) Nagasaki University (Professor Matsuda) Providing information to members of the media regarding verification activities at Nakato Bridge (Saikai City, Nagasaki Prefecture) Explanation of database systems by Tohoku University for managers in the 35 cities, towns, and villages of Yamagata Prefecture (Yamagata City, Yamagata Prefecture) Technology explanatory meeting for members of -

Tsuruoka Koen)
Come to TSURUOKA Yamagata Pref. TSURUOKA Sight-seeing Spots Tsuruoka Urban and Tsuruoka Park Vicinity 37 9 16 10 17 33 18 35 36 19 31 8 5 113 20 4 21 6 1 12 22 40 34 2 3 13 25 14 26 15 23 27 24 7 29 28 30 31 38 42 43 32 39 41 Blue Text: 1. Chido Museum 2. Tsuruoka Park 3. Shonai domain School Chido-kan 4. Kazama’s former residence 5. The Garden of Suge 6. Cathedral 7. Dewashonai International Forum: Amazon Folk Museum 8. Nangakuji Temple Orange Text: 9. Shakitto 10. Tourist Souvenir Shops – Furusato Honpo 11. Togashi Candle Store 12. Ueno School of Goten-ball 13. Yu-kobo 14. Degansu souvenir shop 15. Muraki Folkcraft Black Text: 16. JR Tsuruoka Station 17. Bicycle Rental 18. Han-nyajiTemple 19. Ryukakuji Temple 20. The remains of Nagayama residence 21. Basho’s embarkation spot 22. Original tree of Shonai persimmon 23. Birth place of Chogyu Takayama 24. Tsuruoka Tenmangu Shrine 25. Daitokuji Temple 26. So-onji Temple 27. Nanoka-machi Kannon Temple 28. Inookaji Temple 29. Willow Bend/Deer Bend 30. Komagihara Park 31. Hie Shrine 32. The Birth Memorial for Shuhei Fujisawa 33. Tsuruoka Inter Change 34. Yamagata Highway 35. Old National Route Seven 36. Akagawa River White Text: 37. to Oyama and Yunohama Hot Springs 38. to Yutagawa Hot Springs 39. to Mt Kimbo 40. to Mt Haguro 41. to Kushibiki , Asahi area and Yamagata City 42. Minden 43. Takasaka ©Yamagata Prefecture Tsuruoka Kanko association Dewa Shonai International Forum 1-28 Come to TSURUOKA Yamagata Pref. -

International Online Symposium on Soil C and N Dynamics by Land Use, Management and Climate Changes
International Online Symposium on Soil C and N Dynamics by Land Use, Management and Climate Changes (Local attendance and excursion will be held and live broadcasting in Yamagata, Japan) Main Topics: Carbon sequestration? Greenhouse gases emission? Plants production? Soil fertility? Effect of Climate Changes? Soil mineral and organic components? Date : September 28 - October 01, 2020 Venue: Zoom Cloud meeting for most participants, and Yamagata University for participants who can attend in Tsuruoka, Japan Organizer & Contact: Prof. Weiguo CHENG (Yamagata University, Japan) Phone: +81-235-28-2824 E-mail: [email protected] Sponsor: Yamagata University UGAS, Iwate University Provisional Program: Sep. 28, Morning: Registration, opening, oral communication Lunch: Suzune for local attendees Afternoon: Oral communication Evening: Short oral speech for poster presenter, Gathering party for local attendees Sep. 29, Morning: Oral communication Lunch: Organic food for local attendees Afternoon: Visiting Sakata and LUMC sites with relay live for cloud attendees Evening: Cultural exchange party in Yonohama HS for local attendees Sep. 30, Morning: Visiting Kamo Aquarium and Hagurosan Lunch: Hagurosan vegetarian cuisine Afternoon: Visiting Long-term experiment in Yamagata Evening: Cultural exchange party in Zao HS (Live broadcasting for cloud attendees all day) Oct. 01, Morning: Visiting LUMC near Okama and Yamatera Lunch: on Soba street in Oishida Town Afternoon: Back to Tsuruoka passing Mogami River descent, one route of Oshin movie Evening: Farewell party in Italian restaurant Farinamore (Live broadcasting for cloud attendees all day). -

By Municipality) (As of March 31, 2020)
The fiber optic broadband service coverage rate in Japan as of March 2020 (by municipality) (As of March 31, 2020) Municipal Coverage rate of fiber optic Prefecture Municipality broadband service code for households (%) 11011 Hokkaido Chuo Ward, Sapporo City 100.00 11029 Hokkaido Kita Ward, Sapporo City 100.00 11037 Hokkaido Higashi Ward, Sapporo City 100.00 11045 Hokkaido Shiraishi Ward, Sapporo City 100.00 11053 Hokkaido Toyohira Ward, Sapporo City 100.00 11061 Hokkaido Minami Ward, Sapporo City 99.94 11070 Hokkaido Nishi Ward, Sapporo City 100.00 11088 Hokkaido Atsubetsu Ward, Sapporo City 100.00 11096 Hokkaido Teine Ward, Sapporo City 100.00 11100 Hokkaido Kiyota Ward, Sapporo City 100.00 12025 Hokkaido Hakodate City 99.62 12033 Hokkaido Otaru City 100.00 12041 Hokkaido Asahikawa City 99.96 12050 Hokkaido Muroran City 100.00 12068 Hokkaido Kushiro City 99.31 12076 Hokkaido Obihiro City 99.47 12084 Hokkaido Kitami City 98.84 12092 Hokkaido Yubari City 90.24 12106 Hokkaido Iwamizawa City 93.24 12114 Hokkaido Abashiri City 97.29 12122 Hokkaido Rumoi City 97.57 12131 Hokkaido Tomakomai City 100.00 12149 Hokkaido Wakkanai City 99.99 12157 Hokkaido Bibai City 97.86 12165 Hokkaido Ashibetsu City 91.41 12173 Hokkaido Ebetsu City 100.00 12181 Hokkaido Akabira City 97.97 12190 Hokkaido Monbetsu City 94.60 12203 Hokkaido Shibetsu City 90.22 12211 Hokkaido Nayoro City 95.76 12220 Hokkaido Mikasa City 97.08 12238 Hokkaido Nemuro City 100.00 12246 Hokkaido Chitose City 99.32 12254 Hokkaido Takikawa City 100.00 12262 Hokkaido Sunagawa City 99.13 -

Yamagata Prefecture
Yamagata Prefecture SUZUKI MACHINERY CO., LTD. (Yamagata City) Small type overlock sewing machine having added value MICRON MACHINERY CO., LTD. (Yamagata City) Grinding Solutions, pursuing for nanometer level roundness Shinwa Industrial Ltd. (Shinjo City) Metal plating technology of Shinkansen for rocket Watec Co., Ltd. (Tsuruoka City) Pioneer of ultrasmall CCD camera Prefact Shirata Factory Co., Ltd. (Higashine City) The world's first linear motion bearing without lubricant Hi-MECHA CORPORATION (Yonezawa City) Establishing the global standard of tantalum capacitor manufacturing equipment Oriental Carpet Mills, Ltd. (Higashi-Murayama Gun) The world’s accepted carpets by traditional technology and its own technology 29 SUZUKI MACHINERY CO., LTD. Small type overlock sewing machine having 3-1, Kawarada, Yamagata City Yamagata Prefecture added value Established in 1953 TEL +81-23-684-0843 Shigeo Suzuki http://www.suzuki-ss.co.jp President Ahead of others in the world, it developed the small type overlock sewing machine having the jet-air threading system, automatic thread tension adjustment system and capable of beginners operating easily. Having top share in the field of small type overlock sewing Small type machine in the world overlock sewing Basically small type overlock sewing machine was very complicated machine machine that was requested about 35 points troublesome threading before "Baby Lock" sewing. For the reason of it, it was a source of headache for home use. Suzuki Machinery developed unique system capable of beginners operating easily, and has top share in the filed of small type overlock sewing machine in the world. It has 90% market share of top-class small type overlock sewing machine at over 100,000 yen of retail price in Japan. -

The Damage Situation of and Measures Taken for the Great East Japan Earthquake (100Th Announcement)
This is provisional translation. Please refer to the original text written in Japanese. As of 14:00, September 22, 2011 The damage situation of and measures taken for the Great East Japan Earthquake (100th announcement) Ministry of Health, Labour, and Welfare (MHLW) ※The underlined parts are changes from the last version. 1. Measures taken at MHLW At 14:46 on March 11 (Friday) : The earthquake hit in Sanriku offshore, Miyagi Prefecture. At 14:50 : The Disaster Response Headquarters of MHLW was set up. At 9:00 on March 12 (Saturday) : The Local Liaison Disaster Response Headquarters of MHLW (changed to the Local Disaster Response Headquarters of MHLW) was set up. (Emergency phones were set up.) 2. Disaster information related to MHLW and measures taken by MHLW (1) The Disaster Relief Act Refer to Attachment 1, “The Disaster Relief Act,” for the past developments. ○ Application of the Disaster Relief Act (decisions taken by Prefectural Governors) The Act is applied in all municipalities in Iwate Prefecture, Miyagi Prefecture, and Fukushima Prefecture. The Act is applied in 113 municipalities in other 7 prefectures. ○ Flexible enforcement of the Disaster Relief Act ・ All Prefectural Governments, including those prefectures not affected by the disaster, were notified of the implementation of the flexible enforcement of the Disaster Relief Act, so that even Prefectural Governments not affected by the disaster could actively rescue evacuees. Specifically, it was clarified that when prefectures not affected by the earthquake set up evacuation shelters and temporary housings or rent ryokans (Japanese-style inns) and hotels, a considerable amount of the cost was funded by the Government (from 50 to 90% of the expenses, depending on the financial capability of the affected Local Governments).