P. 1 AC18 Inf. 11 (English Only/ Seulement En Anglais
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Case of Unusual Head Scalation in Vipera Ammodytes
North-Western Journal of Zoology 2019, vol.15 (2) - Correspondence: Notes 195 Castillo, G.N., González-Rivas C.J., Acosta J.C. (2018b): Salvator rufescens Article No.: e197504 (Argentine Red Tegu). Diet. Herpetological Review 49: 539-540. Received: 15. April 2019 / Accepted: 21. April 2019 Castillo, G.N., Acosta, J.C., Blanco, G.M. (2019): Trophic analysis and Available online: 25. April 2019 / Printed: December 2019 parasitological aspects of Liolaemus parvus (Iguania: Liolaemidae) in the Central Andes of Argentina. Turkish Journal of Zoology 43: 277-286. Castillo, G.N., Acosta J.C., Ramallo G., Pizarro J. (2018a): Pattern of infection by Gabriel Natalio CASTILLO1,2,3,*, Parapharyngodon riojensis Ramallo, Bursey, Goldberg 2002 (Nematoda: 1,4 Pharyngodonidae) in the lizard Phymaturus extrilidus from Puna region, Cynthia GONZÁLEZ-RIVAS Argentina. Annals of Parasitology 64: 83-88. and Juan Carlos ACOSTA1,2 Cruz, F.B., Silva, S., Scrocchi, G.J. (1998): Ecology of the lizard Tropidurus etheridgei (Squamata: Tropiduridae) from the dry Chaco of Salta, 1. Faculty of Exact, Physical and Natural Sciences, National University Argentina. Herpetological Natural History 6: 23-31. of San Juan, Av. Ignacio de la Roza 590, 5402, San Juan, Argentina. Goldberg, S.R., Bursey, C.R., Morando, M. (2004): Metazoan endoparasites of 12 2. DIBIOVA Research Office (Diversity and Biology of Vertebrates of species of lizards from Argentina. Comparative Parasitology 71: 208-214. the Arid), National University of San Juan, Av. Ignacio de la Lamas, M.F., Céspedez, J.A., Ruiz-García, J.A. (2016): Primer registro de Roza 590, 5402, San Juan, Argentina. nematodes parásitos para la culebra Xenodon merremi (Squamata, 3. -

Reciprocal Zoos and Aquariums
Reciprocity Please Note: Due to COVID-19, organizations on this list may have put their reciprocity program on hold as advance reservations are now required for many parks. We strongly recommend that you call the zoo or aquarium you are visiting in advance of your visit. Thank you for your patience and understanding during these unprecedented times. Wilds Members: Members of The Wilds receive DISCOUNTED or FREE admission to the AZA-accredited zoos and aquariums on the list below. Wilds members must present their current membership card along with a photo ID for each adult listed on the membership to receive their discount. Each zoo maintains its own discount policies, and The Wilds strongly recommends calling ahead before visiting a reciprocal zoo. Each zoo reserves the right to limit the amount of discounts, and may not offer discounted tickets for your entire family size. *This list is subject to change at any time. Visiting The Wilds from Other Zoos: The Wilds is proud to offer a 50% discount on the Open-Air Safari tour to members of the AZA-accredited zoos and aquariums on the list below. The reciprocal discount does not include parking. If you do not have a valid membership card, please contact your zoo’s membership office for a replacement. This offer cannot be combined with any other offers or discounts, and is subject to change at any time. Park capacity is limited. Due to COVID-19 advance reservations are now required. You may make a reservation by calling (740) 638-5030. You must present your valid membership card along with your photo ID when you check in for your tour. -
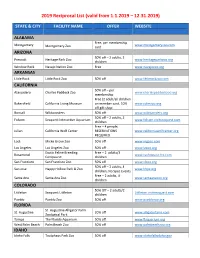
Reciprocal Zoo List 2019 for Website
2019 Reciprocal List (valid from 1.1.2019 – 12.31.2019) STATE & CITY FACILITY NAME OFFER WEBSITE ALABAMA Free, per membership Montgomery www.montgomeryzoo.com Montgomery Zoo card ARIZONA 50% off – 2 adults, 3 Prescott Heritage Park Zoo www.heritageparkzoo.org children Window Rock Navajo Nation Zoo Free www.navajozoo.org ARKANSAS Little Rock Little Rock Zoo 50% off www.littlerockzoo.com CALIFORNIA 50% off – per Atascadero Charles Paddock Zoo www.charlespaddockzoo.org membership Free (2 adult/all children Bakersfield California Living Museum on member card, 10% www.calmzoo.org off gift shop Bonsall Wildwonders 50% off www.wildwonders.org 50% off – 2 adults, 2 Folsom Seaquest Interactive Aquarium www.folsom.visitseaquest.com children Free – 4 people; Julian California Wolf Center RESERVATIONS www.californiawolfcenter.org REQUIRED Lodi Micke Grove Zoo 50% off www.mgzoo.com Los Angeles Los Angeles Zoo 50% off www.lazoo.org Exotic Feline Breeding Free – 2 adults/3 Rosamond www.cathopuise.fcc.com Compound children San Francisco San Francisco Zoo 50% off www.sfzoo.org 50% off – 2 adults, 4 San Jose Happy Hollow Park & Zoo www.hhpz.org children, No Spec Events Free – 2 adults, 4 Santa Ana Santa Ana Zoo www.santaanazoo.org children COLORADO 50% Off – 2 adults/2 Littleton Seaquest Littleton Littleton.visitseaquest.com children Pueblo Pueblo Zoo 50% off www.pueblozoo.org FLORIDA St. Augustine Alligator Farm St. Augustine 20% off www.alligatorfarm.com Zoological Park Tampa The Florida Aquarium 50% off www.flaquarium.org West Palm Beach Palm Beach Zoo 50% off www.palmbeachzoo.org IDAHO Idaho Falls Tautphaus Park Zoo 50% off www.idahofallsidaho.gov 2019 Reciprocal List (valid from 1.1.2019 – 12.31.2019) Free – 2 adults, 5 Pocatello Pocatello Zoo www.zoo.pocatello.us children ILLINOIS Free – 2 adults, 3 Springfield Henson Robinson Zoo children. -

Using Environmental DNA to Detect Estuarine Crocodiles, a Cryptic
Human–Wildlife Interactions 14(1):64–72, Spring 2020 • digitalcommons.usu.edu/hwi Using environmental DNA to detect estuarine crocodiles, a cryptic-ambush predator of humans Alea Rose, Research Institute for Environment and Livelihoods, Charles Darwin University, Darwin, Northern Territory 0909, Australia Yusuke Fukuda, Department of Environment & Natural Resources, Northern Territory Govern- ment, P.O. Box 496, Palmerston, Northern Territory 0831, Australia Hamish A. Campbell, Research Institute of Livelihoods and the Environment, Charles Darwin University, Darwin, Northern Territory 0909, Australia [email protected] Abstract: Negative human–wildlife interactions can be better managed by early detection of the wildlife species involved. However, many animals that pose a threat to humans are highly cryptic, and detecting their presence before the interaction occurs can be challenging. We describe a method whereby the presence of the estuarine crocodile (Crocodylus porosus), a cryptic and potentially dangerous predator of humans, was detected using traces of DNA shed into the water, known as environmental DNA (eDNA). The estuarine crocodile is present in waterways throughout southeast Asia and Oceania and has been responsible for >1,000 attacks upon humans in the past decade. A critical factor in the crocodile’s capability to attack humans is their ability to remain hidden in turbid waters for extended periods, ambushing humans that enter the water or undertake activities around the waterline. In northern Australia, we sampled water from aquariums where crocodiles were present or absent, and we were able to discriminate the presence of estuarine crocodile from the freshwater crocodile (C. johnstoni), a closely related sympatric species that does not pose a threat to humans. -

An Investigation Into Factors Affecting Breeding Success in The
An investigation into factors affecting breeding success in the Tasmanian devil (Sarcophilus harrisii) Tracey Catherine Russell Faculty of Science School of Life and Environmental Science The University of Sydney Australia A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy 2018 Faculty of Science The University of Sydney Table of Contents Table of Figures ............................................................................................................ viii Table of Tables ................................................................................................................. x Acknowledgements .........................................................................................................xi Chapter Acknowledgements .......................................................................................... xii Abbreviations ................................................................................................................. xv An investigation into factors affecting breeding success in the Tasmanian devil (Sarcophilus harrisii) .................................................................................................. xvii Abstract ....................................................................................................................... xvii 1 Chapter One: Introduction and literature review .............................................. 1 1.1 Devil Life History ................................................................................................... -

Suggested Guidelines for Reptiles and Amphibians Used in Outreach
RECOMMENDATIONS FOR REPTILES AND AMPHIBIANS USED IN OUTREACH PROGRAMS Compiled by Diane Barber, Fort Worth Zoo Originally posted September 2003; updated February 2008 INTRODUCTION This document has been created by the AZA Reptile and Amphibian Taxon Advisory Groups to be used as a resource to aid in the development of institutional outreach programs. Within this document are lists of species that are commonly used in reptile and amphibian outreach programs. With over 12,700 species of reptiles and amphibians in existence today, it is obvious that there are numerous combinations of species that could be safely used in outreach programs. It is not the intent of these Taxon Advisory Groups to produce an all-inclusive or restrictive list of species to be used in outreach. Rather, these lists are intended for use as a resource and are some of the more common species that have been safely used in outreach programs. A few species listed as potential outreach animals have been earmarked as controversial by TAG members for various reasons. In each case, we have made an effort to explain debatable issues, enabling staff members to make informed decisions as to whether or not each animal is appropriate for their situation and the messages they wish to convey. It is hoped that during the species selection process for outreach programs, educators, collection managers, and other zoo staff work together, using TAG Outreach Guidelines, TAG Regional Collection Plans, and Institutional Collection Plans as tools. It is well understood that space in zoos is limited and it is important that outreach animals are included in institutional collection plans and incorporated into conservation programs when feasible. -

Gallotia Caesaris, Lacertidae) from Different Habitats Author(S): M
Sexual Size and Shape Dimorphism Variation in Caesar's Lizard (Gallotia caesaris, Lacertidae) from Different Habitats Author(s): M. Molina-Borja, M. A. Rodríguez-Domínguez, C. González-Ortega, and M. L. Bohórquez-Alonso Source: Journal of Herpetology, 44(1):1-12. 2010. Published By: The Society for the Study of Amphibians and Reptiles DOI: 10.1670/08-266.1 URL: http://www.bioone.org/doi/full/10.1670/08-266.1 BioOne (www.bioone.org) is an electronic aggregator of bioscience research content, and the online home to over 160 journals and books published by not-for-profit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Herpetology, Vol. 44, No. 1, pp. 1–12, 2010 Copyright 2010 Society for the Study of Amphibians and Reptiles Sexual Size and Shape Dimorphism Variation in Caesar’s Lizard (Gallotia caesaris, Lacertidae) from Different Habitats 1,2 3 3 M. MOLINA-BORJA, M. A. RODRI´GUEZ-DOMI´NGUEZ, C. GONZA´ LEZ-ORTEGA, AND 1 M. L. BOHO´ RQUEZ-ALONSO 1Laboratorio Etologı´a, Departamento Biologı´a Animal, Facultad Biologı´a, Universidad La Laguna, Tenerife, Canary Islands, Spain 3Centro Reproduccio´n e Investigacio´n del lagarto gigante de El Hierro, Frontera, El Hierro, Canary Islands, Spain ABSTRACT.—We compared sexual dimorphism of body and head traits from adult lizards of populations of Gallotia caesaris living in ecologically different habitats of El Hierro and La Gomera. -

Acrantophis Madagascariensis (Duméril & Bibron, 1844) and A
Kent Academic Repository Full text document (pdf) Citation for published version Gardner, Charlie J. and McDonnell, Naidi and Ellis, Charlotte and Jasper, Louise D. (2017) Observations of aquatic behaviour in Malagasy ground boas Acrantophis madagascariensis (Duméril & Bibron, 1844) and A. dumerili Jan, 1860. Herpetology Notes, 10 . pp. 271-273. DOI Link to record in KAR https://kar.kent.ac.uk/84414/ Document Version Author's Accepted Manuscript Copyright & reuse Content in the Kent Academic Repository is made available for research purposes. Unless otherwise stated all content is protected by copyright and in the absence of an open licence (eg Creative Commons), permissions for further reuse of content should be sought from the publisher, author or other copyright holder. Versions of research The version in the Kent Academic Repository may differ from the final published version. Users are advised to check http://kar.kent.ac.uk for the status of the paper. Users should always cite the published version of record. Enquiries For any further enquiries regarding the licence status of this document, please contact: [email protected] If you believe this document infringes copyright then please contact the KAR admin team with the take-down information provided at http://kar.kent.ac.uk/contact.html 1 Observations of aquatic behaviour in Malagasy ground boas 2 Acrantophis madagascariensis (Duméril & Bibron, 1844) and A. 3 dumerili Jan, 1860 4 5 Charlie J. GardnerI, Naidi McDonnellII, Charlotte EllisII and Louise D. JasperIII 6 7 8 I Durrell Institute of Conservation and Ecology, University of Kent, Canterbury, CT2 7NR, 9 UK 10 II Operation Wallacea, Wallace House, Old Bolingbroke, Spilsby, Lincolnshire, PE23 4EX, 11 UK 12 III Independent Researcher 13 14 Madagascar possesses a diverse snake fauna comprising over 90 species in four families 15 (Jenkins et al. -

Crocodiles Alter Skin Color in Response to Environmental Color Conditions
www.nature.com/scientificreports OPEN Crocodiles Alter Skin Color in Response to Environmental Color Conditions Received: 3 January 2018 Mark Merchant1, Amber Hale2, Jen Brueggen3, Curt Harbsmeier4 & Colette Adams5 Accepted: 6 April 2018 Many species alter skin color to varying degrees and by diferent mechanisms. Here, we show that Published: xx xx xxxx some crocodylians modify skin coloration in response to changing light and environmental conditions. Within the Family, Crocodylidae, all members of the genus Crocodylus lightened substantially when transitioned from dark enclosure to white enclosures, whereas Mecistops and Osteolaemus showed little/no change. The two members of the Family Gavialidae showed an opposite response, lightening under darker conditions, while all member of the Family Alligatoridae showed no changes. Observed color changes were rapid and reversible, occurring within 60–90 minutes. The response is visually- mediated and modulated by serum α-melanocyte-stimulating hormone (α-MSH), resulting in redistribution of melanosomes within melanophores. Injection of crocodiles with α-MSH caused the skin to lighten. These results represent a novel description of color change in crocodylians, and have important phylogenetic implications. The data support the inclusion of the Malayan gharial in the Family Gavialidae, and the shift of the African slender-snouted crocodile from the genus Crocodylus to the monophyletic genus Mecistops. Te rapid alteration of skin color is well known among a wide assortment of ectothermic vertebrates and inver- tebrates1. Adaptive skin color changes may occur for a variety of reasons, including communication, thermal regulation, and crypsis1. Te modifcation of skin pigmentation is achieved by either physiological or morpho- logical mechanisms1. -

Multiple Paternity in a Reintroduced Population of the Orinoco Crocodile (Crocodylus Intermedius) at the El Frío Biological Station, Venezuela
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Online Research @ Cardiff RESEARCH ARTICLE Multiple Paternity in a Reintroduced Population of the Orinoco Crocodile (Crocodylus intermedius) at the El Frío Biological Station, Venezuela Natalia A. Rossi Lafferriere1,2☯*, Rafael Antelo3,4,5☯, Fernando Alda4,6, Dick Mårtensson7, Frank Hailer8,9, Santiago Castroviejo-Fisher10, José Ayarzagüena5†, Joshua R. Ginsberg1,11, Javier Castroviejo5,12, Ignacio Doadrio4, Carles Vilá13, George Amato2 1 Department of Ecology, Evolution and Environmental Biology, Columbia University, New York, New York, United States of America, 2 Sackler Institute of Comparative Genomics, American Museum of Natural History, New York, New York, United States of America, 3 Fundación Palmarito Casanare, Bogotá, Colombia, 4 Dpto. Biodiversidad y Biología Evolutiva, Museo Nacional de Ciencias Naturales, CSIC, Madrid, Spain, 5 Estación Biológica El Frío, Apure, Venezuela, 6 LSU Museum of Natural Science, Department of Biological Sciences, Louisiana State University, Baton Rouge, Louisiana, United States of America, 7 Department of Evolutionary Biology, Uppsala University, Uppsala, Sweden, 8 School of Biosciences, Cardiff University, Cardiff, CF10 3AX, Wales, United Kingdom, 9 Center for Conservation and Evolutionary Genetics, Smithsonian Conservation Biology Institute, National Zoological Park, Washington, DC, United OPEN ACCESS States of America, 10 Lab. de Sistemática de Vertebrados, Pontifícia Universidade Católica do Rio Grande Citation: Rossi Lafferriere NA, Antelo R, Alda F, do Sul (PUCRS), Porto Alegre, Brasil, 11 Cary Institute of Ecosystem Studies, Millbrook, New York, United States of America, 12 Asociación Amigos de Doñana, Seville, Spain, 13 Conservation and Evolutionary Mårtensson D, Hailer F, Castroviejo-Fisher S, et al. -

Phylogenetic Taphonomy: a Statistical and Phylogenetic
Drumheller and Brochu | 1 1 PHYLOGENETIC TAPHONOMY: A STATISTICAL AND PHYLOGENETIC 2 APPROACH FOR EXPLORING TAPHONOMIC PATTERNS IN THE FOSSIL 3 RECORD USING CROCODYLIANS 4 STEPHANIE K. DRUMHELLER1, CHRISTOPHER A. BROCHU2 5 1. Department of Earth and Planetary Sciences, The University of Tennessee, Knoxville, 6 Tennessee, 37996, U.S.A. 7 2. Department of Earth and Environmental Sciences, The University of Iowa, Iowa City, Iowa, 8 52242, U.S.A. 9 email: [email protected] 10 RRH: CROCODYLIAN BITE MARKS IN PHYLOGENETIC CONTEXT 11 LRH: DRUMHELLER AND BROCHU Drumheller and Brochu | 2 12 ABSTRACT 13 Actualistic observations form the basis of many taphonomic studies in paleontology. 14However, surveys limited by environment or taxon may not be applicable far beyond the bounds 15of the initial observations. Even when multiple studies exploring the potential variety within a 16taphonomic process exist, quantitative methods for comparing these datasets in order to identify 17larger scale patterns have been understudied. This research uses modern bite marks collected 18from 21 of the 23 generally recognized species of extant Crocodylia to explore statistical and 19phylogenetic methods of synthesizing taphonomic datasets. Bite marks were identified, and 20specimens were then coded for presence or absence of different mark morphotypes. Attempts to 21find statistical correlation between trace types, marking animal vital statistics, and sample 22collection protocol were unsuccessful. Mapping bite mark character states on a eusuchian 23phylogeny successfully predicted the presence of known diagnostic, bisected marks in extinct 24taxa. Predictions for clades that may have created multiple subscores, striated marks, and 25extensive crushing were also generated. Inclusion of fossil bite marks which have been positively 26associated with extinct species allow this method to be projected beyond the crown group. -

555 Analisis Objek Daya Tarik Wisata Favorit Berdasarkan
ANALISIS OBJEK DAYA TARIK WISATA FAVORIT BERDASARKAN JUMLAH PENGUNJUNG DI DAERAH ISTIMEWA YOGYAKARTA Atun Yulianto NIDN 0505077401 Akademi Pariwisata BSI Yogyakarta E-mail : [email protected] ABSTRACT Besides known as the city with the title of the center of struggle, cultural and educational center, Daerah Istimewa Yogyakarta is also known as a city that has beautiful natural scenery, cultural and traditional arts that are still sustainable until now. Daerah Istimewa Yogyakarta (DIY) has various facilities of tourism with adequate quality and spread across five districts that is Sleman, Kulon Progo, Gunung Kidul, Bantul and Kota Yogyakarta. This study aims to determine the object of tourist attraction in Daerah Istimewa Yogyakarta which is a favorite of tourists based on the number of visitors range from 2011 to 2015. The research method used is descriptive qualitative supported quantitative data to provide a mathematical picture of the three objects of tourist attraction what is the most dominant visited by tourists. The results of this study indicate the order based on the number of visitors, namely Parangtritis Beach occupies the first position, followed by Prambanan Temple and Gembira Loka zoo become the favorite tourist attraction object of visitors.Suggestions in this study is the need for comparative study and adoption of road map of strategic planning and operational in preparing tourism marketing strategy to bring more tourists to the manager of tourist attraction object which is still few number of visitors. Keyword : tourist attraction object, visitors, PENDAHULUAN Luas wilayah daratan DIY adalah Daerah Istimewa Yogyakarta disingkat 3.185,80 km2, atau 0,17 persen dari wilayah DIY dikenal sebagai kota pelajardan budaya.