Application for Inclusion of Gadolinium-Based Contrast Agents to the WHO
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Not for Immediate Release
Press Contact: David Pitts Argot Partners 212.600.1902 [email protected] Bracco Imaging Continues its Investment in Delivering Radiological Solutions Growing Breadth of Portfolio, Research, Education and Innovation Initiatives Highlighted at the 2011 RSNA CHICAGO, November 27, 2011 – Bracco Imaging S.p.A. – one of the world’s leading companies in the diagnostic imaging business and part of the Bracco Group – is highlighting the Company’s investment and commitment to imaging agents and radiology solutions across modalities through its growing product portfolio, as well as research, education and innovation, at the 97th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), which is being held November 27 - December 2, 2011 at Chicago’s McCormick Place, booth #4839. "Bracco remains committed to the diagnostic imaging sector, investing in imaging across modalities, including X-ray, Computed Tomography (CT), Cath Lab, Magnetic Resonance Imaging (MRI), Nuclear Medicine and Ultrasound," according to Fulvio Renoldi Bracco, head of Bracco Imaging, S.p.A. "In a difficult economic climate, where investing in the future is not top of mind, Bracco remains at the forefront. We believe that imaging agents and integrated imaging solutions are critical to support radiologists and other imaging specialists in making an effective diagnosis. Our commitment to providing the market with industry-leading products and solutions, and our partnership with the medical community, therefore remains unchanged." Fulvio Renoldi Bracco concluded: "We perceive this period in the industry, and indeed in the broader markets, as an opportunity to invest and to continue to deliver the best contrast imaging solutions to market. -

The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection
The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Country Reports Japan International Corporation of Welfare Services (JICWELS) Contents 1. Cambodia 1 2. Indonesia 70 3. Malaysia 91 4. Philippines 116 5. Sri Lanka 141 6. Thailand 161 The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Cambodia -1- KINGDOM OF CAMBODIA Nation Religion King Ministry of Health Department of Drugs and Food Country Report The Study Program on Quality Management of Essential Medicines Good Manufacturing Practice (GMP) and Inspection November 4, 2012 – November 30, 2012 Sponsored by : The Government of Japan Japan International Cooperation Agency (JICA) Department of Drugs and Food Ministry of Health, Cambodia. -2- I- COUNTRY PROFILE -3- A-Geography Cambodia is an agricultural country located in South East Asia which bordering the Gulf of Thailand, between Thailand, Vietnam, and Laos. Its approximate geographical coordinates are 13°N 105°E. Its 2,572 km border is split among Vietnam (1,228 km), Thailand (803 km) and Laos (541 km), as well as 443 km of coastline. Cambodia covers 181,035 square kilometers in the southwestern part of the Indochina, Cambodia lies completely within the tropics; its southernmost points are only slightly more than 10° above the equator. The country is bounded on the north by Thailand and by Laos, on the east and southeast by Vietnam, and on the west by the Gulf of Thailand and by Thailand. It consists of the Tonle Sap Basin and the Mekong Lowlands. To the southeast of this great basin is the Mekong Delta, which extends through Vietnam to the South China Sea. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Imaging Diagnostics by Combining Contrast Agents
(19) TZZ Z _T (11) EP 2 907 526 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 19.08.2015 Bulletin 2015/34 A61K 49/04 (2006.01) A61K 51/04 (2006.01) A61K 49/06 (2006.01) (21) Application number: 15155866.5 (22) Date of filing: 20.06.2008 (84) Designated Contracting States: (72) Inventor: Wiebelitz, Ulrike AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 13158 Berlin (DE) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR (74) Representative: Kilger, Ute Boehmert & Boehmert (30) Priority: 22.06.2007 EP 07110922 Anwaltspartnerschaft mbB Patentanwälte Rechtsanwälte (62) Document number(s) of the earlier application(s) in Pettenkoferstrasse 20-22 accordance with Art. 76 EPC: 80336 München (DE) 12199299.4 / 2 572 735 12168380.9 / 2 514 442 Remarks: 08774183.1 / 2 170 405 This application was filed on 20-02-2015 as a divisional application to the application mentioned (71) Applicant: mivenion GmbH under INID code 62. 10115 Berlin (DE) (54) Imaging diagnostics by combining contrast agents (57) The present invention relates to the use of a combination of several contrast agents having different imaging properties with respect to imaging representation. EP 2 907 526 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 2 907 526 A1 2 Description from suspect lesions and to prepare and assess these samples histopathologically. [0001] The present invention relates to the use of a [0005] MRI examination of the female breast has very combination of several contrast agents having different high sensitivity in comparison to other imaging modali- imaging properties. -

Guideline on Core Smpc and Package Leaflet for Gadoteric Acid EMA/CHMP/337820/2016 Page 2/23
1 26 May 2016 2 EMA/CHMP/337820/2016 3 Committee for Medicinal Products for Human Use (CHMP) 4 Guideline on core SmPC and Package Leaflet for gadoteric 5 acid 6 Draft Draft agreed by Radiopharmaceutical Drafting Group April 2016 Adopted by CHMP for release for consultation 26 May 2016 Start of public consultation 1 June 2016 End of consultation (deadline for comments) 30 September 2016 7 Comments should be provided using this template. The completed comments form should be sent to [email protected]. 8 Keywords Magnetic resonance, contrast media, gadolinium compounds, core SmPC, core Package Leaflet, gadoteric acid 9 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. 10 Guideline on core SmPC and Package Leaflet for gadoteric 11 acid 12 Table of contents 13 Executive summary ..................................................................................... 3 14 1. Introduction (background) ...................................................................... 3 15 2. Scope....................................................................................................... 3 16 3. Legal basis .............................................................................................. 3 17 4. Core SmPC and Package Leaflet for gadoteric acid ................................. -

Drug-Induced Anaphylaxis in China: a 10 Year Retrospective Analysis of The
Int J Clin Pharm DOI 10.1007/s11096-017-0535-2 RESEARCH ARTICLE Drug‑induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database Ying Zhao1,2,3 · Shusen Sun4 · Xiaotong Li1,3 · Xiang Ma1 · Huilin Tang5 · Lulu Sun2 · Suodi Zhai1 · Tiansheng Wang1,3,6 Received: 9 May 2017 / Accepted: 19 September 2017 © The Author(s) 2017. This article is an open access publication Abstract Background Few studies on the causes of (50.1%), mucocutaneous (47.4%), and gastrointestinal symp- drug-induced anaphylaxis (DIA) in the hospital setting are toms (31.3%). A total of 249 diferent drugs were involved. available. Objective We aimed to use the Beijing Pharma- DIAs were mainly caused by antibiotics (39.3%), traditional covigilance Database (BPD) to identify the causes of DIA Chinese medicines (TCM) (11.9%), radiocontrast agents in Beijing, China. Setting Anaphylactic case reports from (11.9%), and antineoplastic agents (10.3%). Cephalospor- the BPD provided by the Beijing Center for Adverse Drug ins accounted for majority (34.5%) of antibiotic-induced Reaction Monitoring. Method DIA cases collected by the anaphylaxis, followed by fuoroquinolones (29.6%), beta- BPD from January 2004 to December 2014 were adjudi- lactam/beta-lactamase inhibitors (15.4%) and penicillins cated. Cases were analyzed for demographics, causative (7.9%). Blood products and biological agents (3.1%), and drugs and route of administration, and clinical signs and plasma substitutes (2.1%) were also important contributors outcomes. Main outcome measure Drugs implicated in DIAs to DIAs. Conclusion A variety of drug classes were impli- were identifed and the signs and symptoms of the DIA cases cated in DIAs. -

Radiopharmaceuticals and Contrast Media – Oxford Clinical Policy
UnitedHealthcare® Oxford Clinical Policy Radiopharmaceuticals and Contrast Media Policy Number: RADIOLOGY 034.19 T0 Effective Date: January 1, 2021 Instructions for Use Table of Contents Page Related Policies Coverage Rationale ....................................................................... 1 • Cardiology Procedures Requiring Prior Definitions .................................................................................... 10 Authorization for eviCore Healthcare Arrangement Prior Authorization Requirements .............................................. 10 • Radiation Therapy Procedures Requiring Prior Applicable Codes ........................................................................ 10 Authorization for eviCore Healthcare Arrangement Description of Services ............................................................... 13 • Radiology Procedures Requiring Prior Authorization References ................................................................................... 13 for eviCore Healthcare Arrangement Policy History/Revision Information ........................................... 14 Instructions for Use ..................................................................... 14 Coverage Rationale eviCore healthcare administers claims on behalf of Oxford Health Plans for the following services that may be billed in conjunction with radiopharmaceuticals and/or contrast media: • Radiology Services: Refer to Radiology Procedures Requiring Prior Authorization for eviCore Healthcare Arrangement for additional information. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -
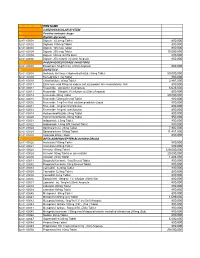
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Magnetic Resonance Imaging (MRI) Contrast Agents Frequently Asked Questions
Magnetic Resonance Imaging (MRI) Contrast Agents Frequently Asked Questions 1. What is an MRI contrast agent? MRI contrast agents are chemical substances that contain the metal gadolinium. They are injected through a vein for many magnetic resonance imaging (MRI) scans. 2. What are the benefits of MRI contrast agents? When injected through a vein into the body, MRI contrast agents improve how internal organs, blood vessels, and other tissues look on the MRI images. This helps detect problems and diagnose medical conditions. For many medical conditions, the use of MRI contrast agents is a necessary part of the MRI. 3. What are the risks from MRI contrast agents? A. Injection problems. Any injection can cause injury to a nerve, artery, or vein, or result in infection. The contrast agent can also sometimes leak outside of the vein into the soft tissues during injection. This is called extravasation and is uncommon (about 1 in every 1,000 injections). This may be painful, but the pain is generally relieved by cold or warm compresses. B. Allergic reaction. Some people can be allergic to MRI contrast agents. There can sometimes be a mild reaction to the contrast agent such as itching and hives (about 1 in every 100 injections). Uncommonly, there can be a serious reaction to the contrast agent such as changes in blood pressure, cardiac distress, and respiratory distress (about 1 in every 10,000 injections). Very rarely this can result in death. For patients who have had a previous mild allergic reaction to an MRI contrast agent and need another MRI with MRI contrast agent, it is generally safe to do so after taking anti-allergy medications. -

Chemical Modifications of Porous Shape Memory Polymers For
molecules Article Chemical Modifications of Porous Shape Memory Polymers for Enhanced X-ray and MRI Visibility Grace K. Fletcher 1, Landon D. Nash 2, Lance M. Graul 1, Lindy K. Jang 1, Scott M. Herting 1, Matthew D. Wilcox 1, Tyler J. Touchet 1, Ana Katarina Sweatt 1, Mary P. McDougall 1,3, Steven M. Wright 1,3 and Duncan J. Maitland 1,2,* 1 Texas A&M University Biomedical Engineering, Bizzell St, College Station, TX 77843, USA; gracekfl[email protected] (G.K.F.); [email protected] (L.M.G.); [email protected] (L.K.J.); [email protected] (S.M.H.); [email protected] (M.D.W.); [email protected] (T.J.T.); [email protected] (A.K.S.); [email protected] (M.P.M.); [email protected] (S.M.W.) 2 Shape Memory Medical Inc., Santa Clara, CA 95054, USA; [email protected] 3 Texas A&M University Electrical and Computer Engineering, Bizzell St, College Station, TX 77843, USA * Correspondence: [email protected] Academic Editor: Irina Savina Received: 31 August 2020; Accepted: 12 October 2020; Published: 13 October 2020 Abstract: The goal of this work was to develop a shape memory polymer (SMP) foam with visibility under both X-ray and magnetic resonance imaging (MRI) modalities. A porous polymeric material with these properties is desirable in medical device development for applications requiring thermoresponsive tissue scaffolds with clinical imaging capabilities. Dual modality visibility was achieved by chemically incorporating monomers with X-ray visible iodine-motifs and MRI visible monomers with gadolinium content. Physical and thermomechanical characterization showed the effect of increased gadopentetic acid (GPA) on shape memory behavior.