Antifungal Activity and Mechanism of the Essential Oils from Litsea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Following Carcinogenic Essential Oils Should Not Be Used In
Aromatherapy Undiluted- Safety and Ethics Copyright © Tony Burfield and Sylla Sheppard-Hanger (2005) [modified from a previous article “A Brief Safety Guidance on Essential Oils” written for IFA, Sept 2004]. Intro In the last 20 years aromatherapy has spread its influence to the household, toiletries and personal care areas: consumer products claiming to relax or invigorate our psyche’s have invaded our bathrooms, kitchen and living room areas. The numbers of therapists using essential oils in Europe and the USA has grown from a handful in the early 1980’s to thousands now worldwide. We have had time to add to our bank of knowledge on essential oils from reflecting on many decades of aromatherapeutic development and history, the collection of anecdotal information from practicing therapists, as well as from clinical & scientific investigations. We have also had enough time to consider the risks in employing essential oils in therapy. In the last twenty years, many more people have had accidents, been ‘burnt’, developed rashes, become allergic, and become sensitized to our beloved tools. Why is this? In this paper, we hope to shed light on this issue, clarify current safety findings, and discuss how Aromatherapists and those in the aromatherapy trade (suppliers, spas, etc.) can interpret this data for continued safe practice. After a refresher on current safety issues including carcinogenic and toxic oils, irritant and photo-toxic oils, we will look at allergens, oils without formal testing, pregnancy issues and medication interactions. We will address the increasing numbers of cases of sensitization and the effect of diluting essential oils. -

Five Hundred Plant Species in Gunung Halimun Salak National Park, West Java a Checklist Including Sundanese Names, Distribution and Use
Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman © 2010 Center for International Forestry Research. All rights reserved. Printed in Indonesia ISBN: 978-602-8693-22-6 Priyadi, H., Takao, G., Rahmawati, I., Supriyanto, B., Ikbal Nursal, W. and Rahman, I. 2010 Five hundred plant species in Gunung Halimun Salak National Park, West Java: a checklist including Sundanese names, distribution and use. CIFOR, Bogor, Indonesia. Photo credit: Hari Priyadi Layout: Rahadian Danil CIFOR Jl. CIFOR, Situ Gede Bogor Barat 16115 Indonesia T +62 (251) 8622-622 F +62 (251) 8622-100 E [email protected] www.cifor.cgiar.org Center for International Forestry Research (CIFOR) CIFOR advances human wellbeing, environmental conservation and equity by conducting research to inform policies and practices that affect forests in developing countries. CIFOR is one of 15 centres within the Consultative Group on International Agricultural Research (CGIAR). CIFOR’s headquarters are in Bogor, Indonesia. It also has offices in Asia, Africa and South America. | iii Contents Author biographies iv Background v How to use this guide vii Species checklist 1 Index of Sundanese names 159 Index of Latin names 166 References 179 iv | Author biographies Hari Priyadi is a research officer at CIFOR and a doctoral candidate funded by the Fonaso Erasmus Mundus programme of the European Union at Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences. -

Olfactometer Screening of Repellent Essential Oils Against the Pollen Beetle (Meligethes Spp.)
Forschungsinstitut für biologischen Landbau Institut de recherche de l’agriculture biologique Research Institute of Organic Agriculture Istituto di ricerche dell’agricoltura biologica Instituto de investigaciones para la agricultura orgánica C. Daniel EXCELLENCE FOR SUSTAINABILITY Research Institute of Organic Agriculture, Ackerstrasse 21, CH-5070 Frick, Switzerland, [email protected] Olfactometer screening of repellent essential oils against the pollen beetle (Meligethes spp.). Odour 1 Odour 2 Air flow Introduction Organic agricultural methods to control pol- in spring. Non-host odors can have repellent len beetle (Meligethes spp.) are limited and effects on pollen beetles (Maucheline 2005). alternatives are needed. The beetles use olfac- We compared the repellent effects of 15 differ- tory cues to locate oilseed rape fields during immigration ent essential oils using a Y-tube-olfactometer. Material and methods Tested essential oils: � Y-tube-olfactometer described by Belz et al. 2013. � Cornmint (Mentha arvensis), � Essential oils diluted 1:10 in acetone; 40 µl applied on filter paper. � Orange (Citrus sinensis), � Filter papers + a flower cluster of oilseed rape were placed in the � Wintergreen (Gaultheria procumbens), odour containers of the olfactometer (control treatment: filter papers � Lemongrass (Cymbopogon flexuosus), with acetone +flower). � Eucalyptus (Eucalyptus globulus), � Beetles were released individually into the olfactometer. � Fir needle (Abies sibirica), � The beetles’ choices were recorded. � Lemon (Citrus limon), � Six replicates with six beetles each were conducted. � Tea-tree (Melaleuca alternifolia), � Repellency values (RV) = nb of beetles on the untreated flower / total � Clove (Syzygium aromaticum), nb of beetles. � Star anise (Illicium verrum), � Wilcoxon signed rank test to test whether mean RV significantly differ- � Grapefruit white (Citrus paradisi), ent from 0.5. -

In Vitro Antibacterial Activity of 34 Plant Essential Oils Against Alternaria Alternata
E3S Web o f Conferences 136, 06006 (2019) https://doi.org/10.1051/e3sconf/20191360 6006 ICBTE 2019 In vitro antibacterial activity of 34 plant essential oils against Alternaria alternata Qiyu Lu1, Ji Liu2, Caihong Tu2, Juan Li3, Chunlong Lei3, Qiliang Guo2, Zhengzhou Zhang2, Wen Qin1* 1College of Food Science and Technology, Sichuan Agricultural University, Ya'an, Sichuan, 625014, China 2Institute of Food Science and Technology, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China 3 Institute of Animal Science, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China Abstract. To determine the antibacterial effect of 34 plant essential oils on Alternaria alternata, 34 plant essential oils such as asarum essential oil, garlic essential oil, and mustard essential oil are used as inhibition agents to isolate A. alternata from citrus as indicator bacteria, through the bacteriostasis test and drug susceptibility test, the types of essential oils with the best inhibitory effect were screened and their concentration was determined. The results showed that the best inhibition effect was mustard essential oil with a minimum inhibitory concentration of 250 μl/L and a minimum bactericidal concentration of 250 μl/L. Followed by the Litsea cubeba essential oil and basil oil, the minimum inhibitory concentration is 500 μl/L. 1 Introduction quality and safety. A. alternata is the main fungus causing post-harvest disease of Pitaya, and the Citrus is a general term for orange, mandarin, orange, antibacterial effect of using cinnamon oil and clove oil is kumquat, pomelo, and medlar. Citrus is the world's good [10]. Clove oil can effectively inhibit the growth of largest fruit, rich in sugar, organic acids, mineral A. -

The Litsea Genome and the Evolution of the Laurel Family
The Litsea genome and the evolution of the laurel family Chen et al 1 Supplementary Note 1. Sample preparation for Litsea cubeba genome sequencing For genome sequencing, we collected buds of L. cubeba. Genomic DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) protocol. For transcriptome analysis, we collected leaves, flowers, and roots from L. cubeba in Zhejiang Province, China, using a karyotype of 2n = 24 (Supplementary Figure 2a). Genome sizes can be determined from the total number of k-mers, divided by the peak value of the k-mer distribution1. To estimate the genome size of L. cubeba, we used a 350 bp pair-end library with 93.08 Gb high-quality reads to calculate the distribution of k-mer values, and found the main peak to be 54 (Supplementary Figure 2b). We estimated the L. cubeba genome size as 1370.14 Mbp, with a 1% heterozygosity rate and a 70.59% repeat sequence, based on an analysis of k-mer-numbers/depths. We used k-mer 41 to obtain a preliminary assembly of L. cubeba, with a scaffold N50 size of 776 bp and a corresponding contig N50 size of 591 bp. Supplementary Note 2. Whole genome duplication analysis in Laurales The KS peaks for WGDs in L. cubeba are both younger (smaller KS values) than the orthologous KS peak between L. cubeba and V. vinifera, implying that the two WGD events are specific to Magnoliids. To compare the WGD peaks of L. cubeba and the speciation events in the lineage of Magnoliids, we performed relative rate tests and corrected orthologous KS peaks between L. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Ethnopharmacological Properties and Medicinal Uses of Litsea Cubeba
plants Review Ethnopharmacological Properties and Medicinal Uses of Litsea cubeba Madhu Kamle 1, Dipendra K. Mahato 2, Kyung Eun Lee 3, Vivek K. Bajpai 4, Padam Raj Gajurel 1, Kang Sang Gu 3,5,* and Pradeep Kumar 1,* 1 Department of Forestry, North Eastern Regional Institute of Science and Technology, Nirjuli 791109, India; [email protected] (M.K.); [email protected] (P.R.G.) 2 School of Exercise and Nutrition Sciences, Deakin University, Burwood, VIC 3125, Australia; [email protected] 3 Molecular Genetics Lab, Department of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Korea; [email protected] 4 Department of Energy and Material Engineering, Dongguk University-Seoul, Seoul 04620, Korea; [email protected] 5 Stemforce, 302 Institute of Industrial Technology, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Korea; [email protected] * Correspondence: [email protected] (K.S.G.); [email protected] (P.K.) Received: 8 May 2019; Accepted: 30 May 2019; Published: 1 June 2019 Abstract: The genus Litsea is predominant in tropical and subtropical regions of India, China, Taiwan, and Japan. The plant possesses medicinal properties and has been traditionally used for curing various gastro-intestinal ailments (e.g., diarrhea, stomachache, indigestion, and gastroenteritis) along with diabetes, edema, cold, arthritis, asthma, and traumatic injury. Besides its medicinal properties, Litsea is known for its essential oil, which has protective action against several bacteria, possesses antioxidant and antiparasitic properties, exerts acute and genetic toxicity as well as cytotoxicity, and can even prevent several cancers. Here we summarize the ethnopharmacological properties, essentials oil, medicinal uses, and health benefits of an indigenous plant of northeast India, emphasizing the profound research to uplift the core and immense potential present in the conventional medicine of the country. -
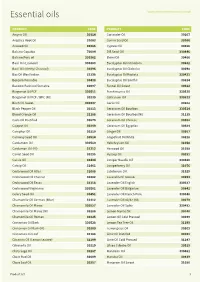
Essential Oils
Essential oils www.inoviainternational.co.uk PRODUCT CODE PRODUCT CODE Amyris Oil 30314 Coriander Oil 30107 Angelica Root Oil 31063 Cumin Seed Oil 30266 Aniseed Oil 30385 Cypress Oil 30256 Balsam Copaiba 70094 Dill Seed Oil 330446 Balsam Peru oil 330362 Elemi Oil 30466 Basil Oil (Linalool) 330483 Eucalyptus Oil Citriodora 30632 Basil Oil (Methyl Chavicol) 30296 Eucalyptus Oil Globulus 30298 Bay Oil West Indian 31398 Eucalyptus Oil Radiata 330421 Benzoin Pourable 30438 Eucalyptus Oil Smithii 30814 Benzoin Resinoid Sumatra 60097 Fennel Oil Sweet 30322 Bergamot Oil FCF 330511 Frankincense Oil 330525 Bergamot Oil FCF / BPC (NI) 30239 Galbanum Oil 330523 Birch Oil Sweet 330437 Garlic Oil 30102 Black Pepper Oil 30313 Geranium Oil Bourbon 330514 Blood Orange Oil 32188 Geranium Oil Bourbon(NI) 31135 Cade Oil Rectified 30679 Geranium Oil Chinese 30302 Cajeput Oil 30249 Geranium Oil Egyptian 30384 Camphor Oil 30110 Ginger Oil 30367 Caraway Seed Oil 30514 Grapefruit Oil White 30228 Cardamom Oil 330510 Helichrysum Oil 31058 Cardamon Oil (NI) 31212 Ho wood Oil 31038 Carrot Seed Oil 30295 Hyssop Oil 30281 Cassia Oil 30434 Juniper Needle Oil 330480 Catnip Oil 31461 Juniperberry Oil 31076 Cedarwood Oil Atlas 31088 Labdanum Oil 31929 Cedarwood Oil Chinese 30282 Lavandin Oil Grosso 30293 Cedarwood Oil Texas 31118 Lavender Oil English 330537 Cedarwood Virginiana 330501 Lavender Oil Bulgarian 30841 Celery Seed Oil 30451 Lavender Oil French Pure 330348 Chamomile Oil German (blue) 31412 Lavender Oil 40/42 (NI) 30279 Chamomile Oil Maroc 330527 Lavender Oil Spike -

1 Repellent Effect of the Caraway Carum Carvi L. on the Rice Weevil Sitophilus Oryzae L
1 Repellent effect of the caraway Carum carvi L. on the rice weevil Sitophilus oryzae L. 2 (Coleoptera, Curculionidae) 3 4 Małgorzata Kłyś1, Aleksandra Izdebska1*, Natalia Malejky-Kłusek1 5 6 1Pedagogical University of Cracow, Institute of Biology, Department of Ecology and 7 Environmental Protection, Podchorążych 2, 30-084 Kraków, Poland 8 e-mail: [email protected] 9 e-mail: [email protected] 10 e-mail: [email protected] 11 * corresponding author 1 12 Abstract 13 Objective 14 The aim of the study was to check whether Carum carvi L. essential oil and L-carvone act on 15 Sitophilus oryzae L. as repellents and/or insecticides, in what concentrations and after what time. 16 Results 17 Caraway essential oil and L-carvone the highest repellency showed not in the highest 18 concentrations used in the tests (1%), but in lower concentrations, respectively 0.5% and 0.1%. 19 Caraway essential oil in all used concentrations showed repellent effects on S. oryzae. The 20 highest repellency (60-98%) caused caraway essential oil in concentration 0.5% after 1, 2, 3, 4 21 and 5 h of the research. The highest repellence of L-carvone (16-100%) resulted in concentration 22 0.1%. The highest mortality of S. oryzae caused 0.5% caraway essential oil. 23 24 Key words: essential oil, insecticides, L-carvone, repellence, storage pests. 25 26 Introduction 27 It is expected that by 2050 the number of people in the world will increase to 9.1 billion and that 28 to feed that number an additional 70% increase in food production will be needed [1, 2, 3]. -

Chemical Composition and Antimicrobial Activity of Selected Essential Oils Against Staphylococcus Spp
antibiotics Article Chemical Composition and Antimicrobial Activity of Selected Essential Oils against Staphylococcus spp. Isolated from Human Semen Miroslava Kaˇcániová 1,2,* , Margarita Terentjeva 3 , Jana Štefániková 4 , Jana Žiarovská 5, Tatsiana Savitskaya 6, Dmitrij Grinshpan 6, Przemysław Łukasz Kowalczewski 7 , Nenad Vukovic 8 and Eva Tvrdá 9 1 Department of Fruit Science, Viticulture and Enology, Faculty of Horticulture and Landscape Engineering, Slovak University of Agriculture, Tr. A. Hlinku 2, 94976 Nitra, Slovakia 2 Department of Bioenergetics, Food Analysis and Microbiology, Institute of Food Technology and Nutrition, University of Rzeszow, Cwiklinskiej 1, 35-601 Rzeszow, Poland 3 Institute of Food and Environmental Hygiene, Faculty of Veterinary Medicine, Latvia University of Life Sciences and Technologies, K. Helman, a iela 8, LV-3004 Jelgava, Latvia; [email protected] 4 AgroBioTech Research Centre, Slovak University of Agriculture, Tr. A. Hlinku 2, 94976 Nitra, Slovakia; [email protected] 5 Department of Plant Genetics and Breeding, Faculty of Agrobiology and Food Resources, Slovak University of Agriculture, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia; [email protected] 6 Research Institute for Physical Chemical Problems, Belarusian State University, Leningradskaya str. 14, 220030 Minsk, Belarus; [email protected] (T.S.); [email protected] (D.G.) 7 Department of Food Technology of Plant Origin, Pozna´nUniversity of Life Sciences, 31 Wojska Polskiego St., 60-624 Pozna´n,Poland; [email protected] 8 Department of Chemistry, Faculty of Science, University of Kragujevac, P.O. Box 12, 34000 Kragujevac, Serbia; [email protected] 9 Department of Animal Physiology, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture, Tr. -

APPLICATION of Litsea Cubeba to IMPROVE SHELF-LIFE of FRESH-CUT ‘PACKHAM’S TRIUMPH’ PEARS
Hrana u zdravlju i bolesti, znanstveno-stručni časopis za nutricionizam i dijetetiku (2016) 5 (2) 48-54 Food in Health and Disease, scientific-professional journal of nutrition and dietetics (2016) 5 (2) 48-54 APPLICATION OF Litsea cubeba TO IMPROVE SHELF-LIFE OF FRESH-CUT ‘PACKHAM’S TRIUMPH’ PEARS Nela Nedić Tiban1*, Hrvoje Pavlović1, Mirela Kopjar1, Marko Bijelić Curkić1, Krunoslav Dugalić2, Vlasta Piližota1 1Josip Juraj Strossmayer University of Osijek, Faculty of Food Technology Osijek, Franje Kuhaca 20, HR-31000 Osijek, Croatia 2Agricultural Institute Osijek, Juzno predgradje 17, HR-31000 Osijek, Croatia Original scientific paper Summary Plant-derived natural products can be of interest as a source of alternatives to improve the shelf-life and the safety of food. Litsea cubeba has recently received much attention due to multiple functions as antibacterial, antifungal, insecticidal, antioxidant and anticancer agent. The application of Litsea cubeba essential oil (LC) in this investigation is one of the first experiments made in order to improve shelf-life of minimally processed fruits. The aim of this work was to investigate the effect of LC at different concentrations (50, 100 and 250 ppm), on shelf-life of fresh-cut pears ‘Packham’s Triumph’ variety. Colour of fresh-cut pears as well as, polyphenol content, antioxidant activity and flesh firmness of untreated and treated samples, were determined. Analysis were carried out immediately following oil treatments, and on 1, 7, 14 day of storage at 2 °C. Treatment with 50 ppm of LC was the most effective treatment to maintain colour of fresh-cut pears during 14 day of storage. -

Protec Botanica Product List
Botanica flo iva flo iva Botanically Inspired Solutions. Naturally. Essential Oils Botanical name Part of plant used Country of origin Floviva Almond Oil Bitter Prunus amygdalus dulcis Kernel USA Floviva Amyris Oil Amyris balsamifera L. Wood Haiti Floviva Angelica Oil Angelica archangelica L. Root / Seed France / Hungary Floviva Aniseed Oil Pimpinella anisum L. Seed Spain / India Floviva Anthopogon Oil Rhododendron anthopogon Leaf & twig Nepal Floviva Basil Oil Ocimum basilicum L. Aerial part Madagascar / Egypt / Vietnam / S. Asia Floviva Bay Laurel Oil Laurus nobilis L. Leaf Albania / Bosnia Herzegovina / C. Europe Floviva Bay Oil WI Pimenta recemosa Leaf Dominica / Jamaica Floviva Benzoin Resinoid Styrax tonkinensis Resin Laos / Thailand Floviva Bergamot Oil Citrus aurantium L. bergamia Peel Calabria, Italy Floviva Bergamot Oil FCF Citrus aurantium L. bergamia Peel Calabria, Italy Floviva Birch Oil Sweet Betula lenta Bark USA Floviva Black Pepper Oil Piper nigrum L. Seed India / Madagascar Floviva Black Spruce Oil Picea mariana Needle Canada Floviva Blue Tansy Oil Tanacetum annuum L. Flower Morocco Floviva Cabreuva Oil Myrocarpus fastigiatus Bark Brazil / Paraguay Floviva Cade Oil Juniperus oxycedrus Wood France / Spain Floviva Cajeput Oil Melaleuca leucadendron L. Leaf & twig Vietnam / Indonesia Floviva Camphor Oil Cinnamomum camphora L. Wood China Floviva Caraway Oil Carum carvi L. Seed Hungary / Russia Floviva Cardamon Oil Elettaria cardamomum L. Seed Guatemala / Sri Lanka / PNG Floviva Carrot Seed Oil Daucus carota L. Seed France Floviva Cassia Oil Cinnamomum cassia L. Bark / Leaf China Floviva Catnip Oil Nepeta cataria Leaf and flower France / USA Floviva Cedarwood Oil Cedrus atlantica Wood Morocco / USA / China Floviva Celery Oil Apium graveolens L.