(Title of the Thesis)*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Energy Systems for Laparoscopic Gynecology Procedures
Available at www.obgmanagement.com S U pp L ement to This supplement is supported by a grant from Ethicon Endo-Surgery, Inc., and has been peer reviewed by the editors of OBG Management. November 2009 Advanced energy systems for laparoscopic gynecology procedures Multifunctional technology yields reliable outcomes, enhances patient safety, and increases procedure efficiency Chair Dr Brill: Probably no surgical instrument defines the gynecolo- gist more than the Kleppinger forceps, the first bipolar device Andrew I. Brill, MD Director of Minimally Invasive used for tubal ligation. It remains an important part of our ar- Gynecology mamentarium despite thermal spread, smoke, char, tissue stick- California Pacific Medical Center ing, and inconsistent hemostasis,1-5 shortcomings that have led San Francisco, California to the development of newer bipolar electrosurgical devices— the LigaSure™ Vessel Sealing System, PlasmaKinetic (PK) plat- Faculty form, and ENSEAL®. These energy-based surgical devices offer John D. Bertrand, MD Director added functionality—coagulation and cutting in a single instru- Minimally Invasive Surgery ment—as well as increased efficiency. These instruments offer Texas Health Dallas specific features that appeal to gynecologic surgeons who have Walnut Hill OB/GYN Associates Dallas, Texas different needs and preferences. Ultrasonic energy technology has also advanced significantly, with instruments such as the Steven D. McCarus, MD Harmonic ACE®, which both cuts and coagulates at the point Chief, Division of Gynecologic Surgery of impact for use in soft-tissue incisions and transections. Director, The Center for Despite our collective surgical experience, the comparative Pelvic Health Florida Hospital Cenebration strengths and weaknesses of these devices have yet to be clearly Orlando, Florida established in an unbiased and reproducible fashion. -

Nurse/Surgical Assistant Quickguide for Baha® FAST Surgery
Nurse/Surgical Assistant Quickguide for Baha® FAST Surgery sound sense for a better life Products in this manual are protected by the following patents: US 5 735 790, US 5 935 170, EP 0715839, EP 0715838 and corresponding patents in other countries and pending patent applications. All products can be subject to change without notice. No part of this publication may be replaced, stored in a retrieval system, or transmitted, in any form by means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the publisher. Caution: Federal law (USA) restricts this device to sale by or on the order of a medical practitioner. ©Entific Medical Systems AB, 2005. All rights reserved. Nurse/Surgical Assistant Quickguide 3 Contents C o n t e n t s • Instruments for set-up 4 • Procedure step by step 6 • Preparation for surgery 8 • Sterilization guidelines 9 • Implantmed sterilization guidelines 11 • Check list 13 Nurse/Surgical Assistant Quickguide 3 Instruments for set-up Instruments for set-up Surgical instruments Pick’n Place instrument set for Baha® The instrument set for Baha® includes instruments for fixture/abutment connection. A complete list of the instruments included is described in detail below. 1. 1. Surgical organizer, titanium 2. Dissector 2. 3. Forceps, titanium 4. Cylinder wrench 3. 5. Screwdriver Unigrip 95 mm 6. Counter torque wrench 4. 7. Drill indicator 8. Connection to handpiece 5. 9. Fixture mount Unigrip (should be placed into titanium organizer) 10. Indicator for Baha 6. 11. Machine screwdriver Unigrip 25 mm 12. Hexagon screwdriver 20 mm 7. 13. Raspatorium 14. -
Fürst Surgical Instruments 1-800-MEDLINE (633-5463) | Medline.Com 1 Experience a New Standard
Fürst Surgical Instruments 1-800-MEDLINE (633-5463) | medline.com 1 Experience a new standard. Choose Fürst for general instrument patterns. Quality, Precision, and Value. Fürst general patterns help reduce instrument costs while maintaining a high level of surgeon satisfaction. Significant savings on routine instrument purchases. A review of purchases made by a health system with 20+ facility locations found that the Fürst line could provide significant savings on over 50% of their annual routine instrument purchases.* Clinically accepted performance. In an independent review, 98.5% of evaluators found Fürst instruments to perform as well as competitive market leaders through extensive clinical use and processing.* Why choose Medline? We’re committed to supporting you. Your Medline Representative is your gateway to everything Fürst—from answering your questions to guiding you through the selection process, and providing you with samples. Fürst’s Lifetime Warranty. We warranty every Fürst surgical instrument to be free of functional defects in workmanship and materials. Medline promises to repair or replace, free of charge, any surgical instrument that does not meet these requirements when used for its intended surgical purpose. 2 MEDLINE 1-800-MEDLINE (633-5463) | medline.com 3 Inches 10 10 250 240 230 9 9 Knife Handles 220 No. 3 Knife Handles Item No. Length Description 210 MDS0610300F 5" (12.7 cm) For blades 10-12, 15 8 MDS0610301F 5" (12.7 cm) Engraved ruler. For blades 10-12, 15. 8 200 190 No. 3K Knife Handle Round. Knurled handle. For blades 10-12, 15. 180 7 Item No. Length 7 MDS0190694F 4" (10.2 cm) 170 No. -

Care and Maintenance of Tissue and Dressing Forceps
Instrument Whisperer by Rick Schultz Care and Maintenance of Tissue and Dressing Forceps LTHOUGH TISSUE AND Q: What are atraumatic forceps? INSPECTING FORCEPS dressing forceps have a A: Atraumatic forceps are designed to Forcep inspection begins at the proximal relatively basic design, they minimize damage and trauma to the end, where the forcep is held. Inspect the require careful inspection to tissue. The most common styles are base of the forcep where the two halves Aensure surgeon satisfaction and patient Cooley and Debakey. are joined. Cracks commonly occur at safety. It is important to first understand the rivet and move to the edge. Inspect the answers to the following frequently the handle and tips for blood and tissue. asked questions. Dressing forceps must be inspected in the serrations, while the teeth of tissue forceps Q: What is the difference between tissue must be closely examined for bioburden. forceps and dressing forceps? Finally, inspect the distal tips to ensure A: Tissue forceps have teeth (e.g., 1x2, Q: The Operating Room (OR) places they meet evenly and don’t overlap. If 2x3, 3x4) that are designed to grasp forceps on the side of the tray. Is this the tips are out of alignment, remove the and manipulate tissue. Dressing forceps acceptable? forcep from the tray and send it out for have serrations instead of teeth, and A: Yes. However, placing forceps on the repair. are designed to grasp and hold gauze side of the tray is a practice occasionally Tissue Forcep Dressing Forcep and dressing during a surgical procedure. used in the OR to organize and quickly pass the forcep to the sterile field. -

Paper No. 1 UNITED STATES PATENT and TRADEMARK OFFICE
Paper No. 1 UNITED STATES PATENT AND TRADEMARK OFFICE _______________ BEFORE THE PATENT TRIAL AND APPEAL BOARD _____________ AURIS HEALTH, INC. Petitioner, v. INTUITIVE SURGICAL OPERATIONS, INC. Patent Owner. Patent No. 6,491,701 _______________ Inter Partes Review No. IPR2019-01532 ____________________________________________________________ Petition for Inter Partes Review of U.S. Patent No. 6,491,701 IPR2019-01532 TABLE OF CONTENTS I. INTRODUCTION ............................................................................................1 II. REGULATORY INFORMATION ................................................................ 2 A. CERTIFICATION PETITIONER MAY CONTEST THE ’701 PATENT (§ 42.104(A)) 2 B. IDENTIFICATION OF CHALLENGED CLAIMS (§ 42.104(B)) ............................... 3 C. FEE FOR INTER PARTES REVIEW (§ 42.15(A)) .................................................. 3 III. BACKGROUND ..........................................................................................3 A. BACKGROUND TECHNOLOGY ..........................................................................3 B. SUMMARY OF THE ’701 PATENT ......................................................................5 C. SUMMARY OF THE ’701 PATENT’S PROSECUTION HISTORY ............................ 8 D. PERSON OF ORDINARY SKILL IN THE ART ....................................................... 9 IV. CLAIM CONSTRUCTION ......................................................................10 A. “END EFFECTOR” (ALL CHALLENGED CLAIMS) .............................................11 -

Original Article
J. Dhaka National Med. Coll. Hos. 2011; 17 (01): 25-28 Original Article Prospective Study of Harmonic Scalpel in about 800 cases covering both open and laparoscopic methods. A.M. Shahidulla1, Md. Aminul Islam2, Zakaria Pavreen3 1Professor and Head, Department of Surgery, Dhaka Community Medical College, 2Associate Professor, Department of Anaesthesiology, Dhaka National Medical College, 3Consultant, Department of Gynae & Obst., Diabetic Center, Narayangonj. Abstract Harmonic (ultracision) Scalpel is a part & parcel of modern Day surgery, Endo-laparoscopic & minimally access surgery. We use this instrument in different minimally access surgeries for the last 5 years. Different open & laparoscopic operations of about 800 cases were performed within this period. Results of our operations with Harmonic Scalpel were very much inspiring, safe and surgeon-friendly with a rapid discharge of the patient from the hospital. The main disadvantages are that the instrument itself is costly & so is its maintenance. It needs a long term practice to be handy. Among open surgeries breast, thyroid, hemorrhoids, tonsils etc. are to be mentioned. In laparoscopic surgery, Harmonic scalpel reduced total operation time in our series. Harmonic scalpel is a laparoscopic surgical instrument that is changing doctors’ performance as well as decreasing patient’s recovery time. Although, it is not poor-friendly, but can be used for safe, rapid surgery & patient-friendly operation. Keywords: Harmonic scalpel, minimally access surgery. coagulum. Harmonic scalpel is indicated for soft tissue Introduction incisions when bleeding control & minimal thermal Harmonic Scalpel- the instrument which utilizes injury are desired. It is used both in open & ultrasonic energy to enable haemostatic cutting &/or endoscopic surgical procedures producing less coagulation of soft tissue. -

Surgical Product Catalog
Surgical Product Catalog ORDERING Call toll free 1.(800) 979.2020 US and Canada (610) 889.0200 International +31 (0) 485.350300 Europe Fax: (610) 889.3233 [email protected] 3222 Phoenixville Pike, Building #50 • Malvern, PA 19355 USA European Office: Simon Homburgstraat 21 • 5431 NN, Cuijk, NL About Us Accutome is entering our fourth decade in the ophthalmic industry. The three major traits that keep the Accutome brand growing remain the same: superior customer service, quality, innovation. It is our goal to make every experience you have with us special and productive. Our focus is to continue to improve our product line and customer service to better suit your needs. We are constantly adding more customer service and sales representatives to provide worldwide support. We also have a European office in the Netherlands. We have become a total solutions provider for all of your ophthalmic needs by supplying quality products such as office medications, supplies, surgical instruments, and diagnostic equipment. We carry thousands of products to meet your needs. If you do not see a particular item, give our representatives a call, and we will find it for you. Accutome Surgical Instruments and Supplies Accutome offers a full line of diamond and metal surgical blades along with handheld surgical instruments and surgical disposable products. We are one of the top production and repair facilities of diamond knives. We cross-reference and competitively bid on all major brands of ophthalmic instruments - just send us your list for a quote. Our reputation for innovation has been developed based on our relationships with top surgeons. -

SECUROS Surgical Instrument Guide Instrument Surgical
SECUROS Surgical Instrument Guide SECUROS Surgical SECUROS Surgicalinstruments Instrument Guide SECUROS USA SECUROS Europe GmbH SECUROS UK 443 Main Street Take Off Gewerbepark 4 Admail 4017 Fiskdale, MA 01518 78579 Neuhausen Ob Eck Germany Coventry, CV1 1YS Tel: (877) BONEFIX (266-3349) Tel.: +49 7467 9476 50 England Fax: (508) 347-5330 Fax: +49 7467 9476 52 Tel: +0845 070 2498 Email: [email protected] Email: [email protected] Fax:+0845 070 2499 www.securos.com www.securos-europe.eu Email: [email protected] www.securos.co.uk Where imagination becomes reality. ©2011 SECUROS. All rights reserved. This page is intentionally left blank. SECUROS Surgical Instrument Guide Table of Contents Instrument Care & Repair 2 Scapel Handles & Blades 14 Scissors 18 Forceps 32 Hemostats 40 Needle Holders 54 Retractors 62 Rongeurs & Bone Forceps 72 Orthopedics 80 Miscellaneous 106 Ophthalmic 116 Dental 124 Instrument Packs 134 Index 138 SECUROS cleanersInstrument Care & Repair Warranty................................................................................. 3 Maintenance.&.Repair.Information.......................................... 4 Troubleshooting.................................................................5-10 Detailed.Instrument.Inspection..........................................8-10 ColorDot............................................................................... 11 Cleaners..........................................................................12-14 2 SECUROS.Surgical.Instrument.Guide.|.1.(877).BONEFIX.(266-3349).|.www.SECUROS.com -

Instrument Measuring: Why Precise Descriptions Are Necessary When Completing Count Sheets
INSTRUMENT WHISPERER Instrument Measuring: Why Precise Descriptions Are Necessary When Completing Count Sheets BY RICK SCHULTZ sing descriptions such as “small,” “medium,” “large,” “heavy,” “long,” etc. is not an acceptable unit of measure Uwhen noting the size of an instrument on an instrument count sheet. The science of surgery requires the precise selection of specific instruments for each individual, surgical tray and procedure. A “medium” scissor, in the eye of one technician or surgeon, may mean a completely different size scissor to another. Just as a baking recipe should be carefully written and followed, the same can be said of every instrument count sheet. A recipe would never call for a handful of flour or a medium cup of Figure 1 Figure 2 sugar, just as an instrument count sheet should never simply describe a retractor as “large.” Each instrument description assembling the trays should be taught A. Jaw angle; should include the instrument’s full how to measure these scissors (See B. Shaft length; name, as well as the length or size, Figure 1). The sharpness test standard for C. Bite size; and measured in centimeteres/millimeters or vascular scissors is yellow scissor testing D. Bite opening inches. material. It is important to use the following Kerrison rongeurs are usually only The sharpness test standard for measurement tips as a guide for verifying referred to by their bite size, 1 mm thru Kerrison rongeurs is to cut cleanly the size of certain instruments. 6 mm (See Figures 2 and 3). This bite through one thickness of an index card Vascular scissors are designed in size reference is only 25% of the specific or 100# card stock, without tearing. -

Surgical Instruments
BR SURGICAL • SURGICAL PROCEDURE EXPERTS INSTRUMENTS | SETS ENDOSCOPIC VIDEO EQUIPMENT OVER 20,000 SURGICAL INSTRUMENTS & PROCEDURE SETS ENDOSCOPIC VIDEO EQUIPMENT & RELATED MEDICAL DEVICES SURGICAL BR SURGICAL, LLC • 3500 Beachwood Court • Suite 107 • Jacksonville, Florida 32224 Office: (904) 642-1366 • Fax: (904) 642-1368 • Toll Free: (888) 642-1366 INSTRUMENTS www.brsurgical.com OVER 20,000 SURGICAL INSTRUMENTS & PROCEDURE SETS ENDOSCOPIC VIDEO EQUIPMENT & RELATED MEDICAL DEVICES BR SURGICAL, LLC F Precision crafted German stainless steel instruments F Surgical instrument pattern is equal to or better than industry standard F All instruments put through our stringent inspection process to ensure consistent high quality F Continuous quality improvements F INDUSTRY BEST WARRANTY Surgical instruments are guaranteed to be free of defects in materials and workmanship for the life of the instrument F FREE LIFETIME SHARPENING on Gold Series biopsy punches Customer sends the biopsy punch with return address to BR Surgical and we take care of the rest F Customer issues handled to their complete satisfaction Scan this QR Code with your smartphone or tablet and connect with us online! BR SURGICAL, LLC • 3500 Beachwood Court • Suite 107 • Jacksonville, Florida 32224 Office: (904) 642-1366 • Fax: (904) 642-1368 • Toll Free: (888) 642-1366 www.brsurgical.com CONTENTS Instrument Care . .2 Repair Service . 3 Limited Return Policy . .3 Custom Instruments . .3 Warranty Cross Reference Information . 3 Endoscope Cleaning & Sterilizing Information . .4 Hysteroscopy Video System . .6 BR Surgical, LLC Hysteroscopy Accessories . .7 instruments are Cystoscopy Video System . 8 ENT/Sinus Video System . 9 guaranteed to be Flexible Rhinolaryngoscope Video System . 10 free of defects Flexible Rhinolaryngoscope . -
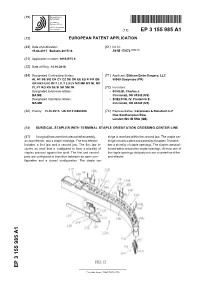
Surgical Stapler with Terminal Staple Orientation Crossing Center Line
(19) TZZ¥__T (11) EP 3 155 985 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 19.04.2017 Bulletin 2017/16 A61B 17/072 (2006.01) (21) Application number: 16193975.6 (22) Date of filing: 14.10.2016 (84) Designated Contracting States: (71) Applicant: Ethicon Endo-Surgery, LLC AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 00969 Guaynabo (PR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (72) Inventors: Designated Extension States: • SCHEIB, Charles J. BA ME Cincinnati, OH 45242 (US) Designated Validation States: • SHELTON, IV, Frederick E. MA MD Cincinnati, OH 45242 (US) (30) Priority: 15.10.2015 US 201514884096 (74) Representative: Carpmaels & Ransford LLP One Southampton Row London WC1B 5HA (GB) (54) SURGICAL STAPLER WITH TERMINAL STAPLE ORIENTATION CROSSING CENTER LINE (57) A surgical instrument includes a shaft assembly, tridge is received within the second jaw. The staple car- an end effector, and a staple cartridge. The end effector tridge includes a deck and a plurality of staples. The deck includes a first jaw and a second jaw. The first jaw in- has a plurality of staple openings. The staples are posi- cludes an anvil that is configured to form a plurality of tioned within respective staple openings. At least one of staples pressed against the anvil. The first and second the staple openings obliquely crosses a centerline of the jaws are configured to transition between an open con- end effector. -

Sterilcontainer System Catalog
SterilContainer™ System General Catalog Aesculap Surgical Technologies — Sterile Processing Table of Contents Introduction ■ History ..................................................................................................... 4-6 ■ SterilContainer™ Design ................................................................... 7-8 SterilContainer System Containers & Baskets .................................... 9-23 ■ Full-Size .................................................................................................. 10-11 ■ Three-Quarter Size ............................................................................... 12-13 ■ Half Size .................................................................................................. 14-15 ■ Extra-Long Mini Size ........................................................................... 16 ■ Extra-Long Size ..................................................................................... 17 ■ Quarter Size ........................................................................................... 18-19 ■ Mini Size ................................................................................................. 20 ■ Wide-Body Size ..................................................................................... 21 ■ SterilContainer™ S ............................................................................... 22-23 Instrument Organization System ............................................................. 25-41 General Accessories ......................................................................................