APHIS Actions (Updated August 3, 2021) TAG No. P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna Lepidopterologica Volgo-Uralensis" 150 Years Later: Changes and Additions
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2000) 31 (1/2):327-367< Würzburg, ISSN 0171-0079 "Fauna lepidopterologica Volgo-Uralensis" 150 years later: changes and additions. Part 5. Noctuidae (Insecto, Lepidoptera) by Vasily V. A n ik in , Sergey A. Sachkov , Va d im V. Z o lo t u h in & A n drey V. Sv ir id o v received 24.II.2000 Summary: 630 species of the Noctuidae are listed for the modern Volgo-Ural fauna. 2 species [Mesapamea hedeni Graeser and Amphidrina amurensis Staudinger ) are noted from Europe for the first time and one more— Nycteola siculana Fuchs —from Russia. 3 species ( Catocala optata Godart , Helicoverpa obsoleta Fabricius , Pseudohadena minuta Pungeler ) are deleted from the list. Supposedly they were either erroneously determinated or incorrect noted from the region under consideration since Eversmann 's work. 289 species are recorded from the re gion in addition to Eversmann 's list. This paper is the fifth in a series of publications1 dealing with the composition of the pres ent-day fauna of noctuid-moths in the Middle Volga and the south-western Cisurals. This re gion comprises the administrative divisions of the Astrakhan, Volgograd, Saratov, Samara, Uljanovsk, Orenburg, Uralsk and Atyraus (= Gurjev) Districts, together with Tataria and Bash kiria. As was accepted in the first part of this series, only material reliably labelled, and cover ing the last 20 years was used for this study. The main collections are those of the authors: V. A n i k i n (Saratov and Volgograd Districts), S. -

Long-Term Strategy for Russian Olive and Saltcedar Management
LONG-TERM STRATEGY FOR RUSSIAN OLIVE AND SALTCEDAR MANAGEMENT YELLOWSTONE RIVER CONSERVATION DISTRICT COUNCIL Prepared by Thomas L. Pick, Bozeman, Montana May 1, 2013 Cover photo credit: Tom Pick. Left side: Plains cottonwood seedlings on bar following 2011 runoff. Top: Saltcedar and Russian olive infest the shoreline of the Yellowstone River between Hysham and Forsyth, Montana. Bottom: A healthy narrowleaf cottonwood (Populus angustifolia James) stand adjacent to the channel provides benefits for wildlife and livestock in addition to bank stability and storing floodwater for later release. Table of Contents page number Agreement ………………………………………………………………………………………………………………………………………….1 Executive Summary …………………………………………………………………………………………………………………………….2 Long-Term Strategy for Russian Olive and Saltcedar Management 1.0 Introduction ………………………………………………………………………………………………………………………4 1.1 Biology and Ecology of Russian Olive and Saltcedar ……………………………………………………5 1.12 Russian Olive 1.13 Saltcedar 1.2 Distribution and Spread……………………………………………………………………………………………..6 1.3 Summary of Impacts ………………………………………………………………………………………………….6 1.4 Legal Framework ……………………………………………………………………………………………………….7 2.0 Strategic Management Objective and Goals ………………………………………………………………..…..8 2.1 Goal 1: Prevent New Infestations (Control Spread) …………………………………….……….……8 2.2 Goal 2: Eradicate All Infestations Within the River Corridor ………………………………………9 2.3 Goal 3: Manage Populations Outside of the River Corridor ……………………………………….9 3.0 Treatment Strategies and Priorities …………………………………….……………………………………………9 -

Final Report
Final Report Final pre-release investigations of the gorse thrips (Sericothrips staphylinus) as a biocontrol agent for gorse (Ulex europaeus) in North America Date: August 31, 2012 Award Number: 10-CA-11420004-184 Report Period: June 1, 2010– May 31, 2012 Project Period: June 1, 2010– May 31, 2012 Recipient: Oregon State University Recipient Contact Person: Fritzi Grevstad Principal Investigator/ Project Director: Fritzi Grevstad Introduction Gorse (Ulex europaeus) is an environmental weed classified as noxious in the states of Washington, Oregon, California, and Hawaii. A classical biological control program has been applied in Hawaii with the introduction of 4 gorse-feeding arthropods, but only two of these (a mite and a seed weevil) have been introduced to the mainland U.S. The two insects that have not yet been introduced include the gorse thrips, Sericothips staphylinus (Thysanoptera: Thripidae), and the moth Agonopterix umbellana (Lepidoptera: Oecophoridae). With prior support from the U.S. Forest Service (joint venture agreement # 07-JV-281), we were able to complete host specificity testing of S. staphylinus on 44 North American plant species that were on the original test plant list. However, following review of the proposed Test Plant List, the Technical Advisory Group on Biocontrol of Weeds (TAG) recommended that we include an additional 18 plant species for testing. In this report, we present host specificity testing and related objectives necessary to bring the program to the implementation stage. Objectives (1) Acquire and grow the additional 18 species of plants recommended by the TAG. (2) Complete host specificity trials for the gorse thrips on the 18 plant species. -

Phaeoseptaceae, Pleosporales) from China
Mycosphere 10(1): 757–775 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/17 Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China Liu NG1,2,3,4,5, Hyde KD4,5, Bhat DJ6, Jumpathong J3 and Liu JK1*,2 1 School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China 2 Guizhou Key Laboratory of Agricultural Biotechnology, Guizhou Academy of Agricultural Sciences, Guiyang 550006, P.R. China 3 Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand 4 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 5 Mushroom Research Foundation, Chiang Rai 57100, Thailand 6 No. 128/1-J, Azad Housing Society, Curca, P.O., Goa Velha 403108, India Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK 2019 – Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10(1), 757–775, Doi 10.5943/mycosphere/10/1/17 Abstract A new hyphomycete genus, Pleopunctum, is introduced to accommodate two new species, P. ellipsoideum sp. nov. (type species) and P. pseudoellipsoideum sp. nov., collected from decaying wood in Guizhou Province, China. The genus is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells and muriform, oval to ellipsoidal conidia often with a hyaline, elliptical to globose basal cell. Phylogenetic analyses of combined LSU, SSU, ITS and TEF1α sequence data of 55 taxa were carried out to infer their phylogenetic relationships. The new taxa formed a well-supported subclade in the family Phaeoseptaceae and basal to Lignosphaeria and Thyridaria macrostomoides. -

Download Full Article in PDF Format
Cryptogamie, Mycologie, 2013, 34 (4): 303-319 © 2013 Adac. Tous droits réservés Phylogeny and morphology of Leptosphaerulina saccharicola sp. nov. and Pleosphaerulina oryzae and relationships with Pithomyces Rungtiwa PHOOKAMSAK a, b, c, Jian-Kui LIU a, b, Ekachai CHUKEATIROTE a, b, Eric H. C. McKENZIE d & Kevin D. HYDE a, b, c * a Institute of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand b School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand c International Fungal Research & Development Centre, Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, Yunnan, 650224, China d Landcare Research, Private Bag 92170, Auckland, New Zealand Abstract – A Dothideomycete species, associated with leaf spots of sugarcane (Saccharum officinarum), was collected from Nakhonratchasima Province, Thailand. A single ascospore isolate was obtained and formed the asexual morph in culture. ITS, LSU, RPB2 and TEF1α gene regions were sequenced and analyzed with molecular data from related taxa. In a phylogenetic analysis the new isolate clustered with Leptosphaerulina americana, L. arachidicola, L. australis and L. trifolii (Didymellaceae) and the morphology was also comparable with Leptosphaerulina species. Leptosphaerulina saccharicola is introduced to accommodate this new collection which is morphologically and phylogenetically distinct from other species of Leptosphaerulina. A detailed description and illustration is provided for the new species, which is compared with similar taxa. The type specimen of Pleosphaerulina oryzae, is transferred to Leptosphaerulina. It is redescribed and is a distinct species from L. australis, with which it was formerly synonymized. Leptosphaerulina species have been linked to Pithomyces but the lack of phylogenetic support for this link is discussed. -

Alhagi Maurorum
Prepared By Jacob Higgs and Tim Higgs Class 1A EDRR- Early Detection Rapid Response Watch List Common crupina Crupina vulgaris African rue Peganum harmala Small bugloss Anchusa arvensis Mediterranean sage Salvia aethiopis Spring millet Milium vernale Syrian beancaper Zygophyllum fabago North Africa grass Ventenata dubia Plumeless thistle Carduus acanthiodes Malta thistle Centaurea melitensis Common Crupina Crupina vulgaris African rue Peganum harmala Small bugloss Anchusa arvensis Mediterranean sage Salvia aethiopis Spring millet Milium vernale Syrian beancaper Zygophyllum fabago North Africa grass Ventenata dubia Plumeless thistle Carduus acanthiodes Malta thistle Centaurea melitensis m Class 1B Early Detection Camelthorn Alhagi maurorum Garlic mustard Alliaria petiolata Purple starthistle Cantaurea calcitrapa Goatsrue Galega officinalis African mustard Brassica tournefortii Giant Reed Arundo donax Japanese Knotweed Polygonum cuspidatum Vipers bugloss Echium vulgare Elongated mustard Brassica elongate Common St. Johnswort Hypericum perforatum L. Oxeye daisy Leucanthemum vulgare Cutleaf vipergrass Scorzonera laciniata Camelthorn Alhagi maurorum Garlic mustard Alliaria petiolata Purple starthistle Cantaurea calcitrapa Goatsrue Galega officinalis African mustard Brassica tournefortii Giant Reed Arundo donax Japanese Knotweed Polygonum cuspidatum Vipers bugloss Echium vulgare Elongated mustard Brassica elongate Common St. Johnswort Hypericum perforatum L. Oxeye daisy Leucanthemum vulgare Cutleaf vipergrass Scorzonera laciniata Class 2 Control -
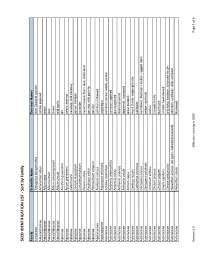
SEED IDENTIFICATION LIST - Sort by Family
SEED IDENTIFICATION LIST - Sort by Family Family Scientific Name Common Names Aizoaceae Tetragonia tetragonoides New Zealand spinach Amaranthaceae Amaranthus albus tumble pigweed Amaryllidaceae Allium cepa onion Amaryllidaceae Allium porrum leek Amaryllidaceae Allium schoenoprasum chives Amaryllidaceae Allium vineale wild garlic Apiaceae Anethum graveolens dill Apiaceae Apium graveolens celery, celeriac Apiaceae Carum carvi caraway; wild caraway Apiaceae Conium maculatum poison hemlock Apiaceae Coriandrum sativum coriander Apiaceae Daucus carota carrot; Queen Ane's lace; wild carrot Apiaceae Pastinaca sativa parsnip; wild parsnip Apiaceae Petroselinum crispum parsley Apocynaceae Asclepias syriaca common milkweed Asparagaceae Asparagus officinalis asparagus Asteraceae Achillea millefolium common yarrow, woolly yarrow Asteraceae Ambrosia artemisiifolia common ragweed Asteraceae Ambrosia trifida giant ragweed Asteraceae Anthemis arvensis field chamomile Asteraceae Anthemis cotula dogfennel, mayweed Asteraceae Arctium lappa great burdock Asteraceae Carduus nutans musk thistle, nodding thistle Asteraceae Carthamus tinctorius safflower Asteraceae Centaurea cyanus cornflower, bachelor's button, ragged robin Asteraceae Centaurea solstitialis yellow starthistle Asteraceae Cichorium endivia endive Asteraceae Cirsium arvense Canada thistle Asteraceae Cirsium vulgare bull thistle Asteraceae Crepis capillaris smooth hawksbeard Asteraceae Cynara cardunculus artichoke, cardoon, artichoke thistle Asteraceae Helianthus annuus (all types, cultivated and -

Current and Potential Use of Phytophagous Mites As Biological Control Agent of Weeds
Chapter 5 Current and Potential Use of Phytophagous Mites as Biological Control Agent of Weeds Carlos Vásquez, Yelitza Colmenárez, José Morales-Sánchez, Neicy Valera, María F. Sandoval and Diego Balza Additional information is available at the end of the chapter http://dx.doi.org/10.5772/59953 1. Introduction Biological control of weeds by using phytophagous mites may help to contain infestations and reduce their spread in time. Although, eradication is not the goal due to the vastness of the areas, the most desirable scenario is achieved when weeds are no longer a concern and no other control is necessary. However, biological control should not be considered the unique strategy to face weed problems, thus commonly; other methods are still required to attain the desired level of control. There is an increasingly interest in using mites for biological control of weeds, primarily those belonging to Eriophyidae because of they are host-specific and often weaken the host plant affecting plant growth and reproduction. Although eriophyid mite species impact the fitness of their host plant, it is not clear how much they have contributed to reduction of the population of the target weed. In some cases, natural enemies, resistant plant genotypes, and adverse abiotic conditions have reduced the ability of eriophyid mites to control target weed popula‐ tions. Besides, susceptibility of eriophyids to predators and pathogens may also prevent them from achieving population densities necessary to reduce host plant populations. In addition to eriophyid mites, tetranychid mites are also being considered as an alternative for weed control. The gorse spider mite, Tetranychus lintearius Dufour, has shown to reduce shoot growth on gorse (Ulex europaeus L.) by around 36% in impact studies conducted over 2.5 years in Tasmania. -

Field Evaluation of a Gall Mite for Biological Control of False Cleavers
Field Evaluation of a Gall Mite for Biological Control of False Cleavers Canola Agronomic Research Program Project #AG-2002-20 Final Report A.S. McClay, Ph.D. McClay Ecoscience 15 Greenbriar Crescent Sherwood Park, AB T8H 1H8 TABLE OF CONTENTS LIST OF TABLES.......................................................................................................................... ii LIST OF FIGURES .......................................................................................................................iii 1 Abstract................................................................................................................................... 1 2 Background and Objectives .................................................................................................... 1 3 Experimental Methods............................................................................................................ 2 3.1 Importation...................................................................................................................... 2 3.2 Colony establishment...................................................................................................... 3 3.3 Virus testing.................................................................................................................... 4 3.4 Canola plots.................................................................................................................... 4 3.5 Dispersal plots................................................................................................................ -

Phragmites Australis
Journal of Ecology 2017, 105, 1123–1162 doi: 10.1111/1365-2745.12797 BIOLOGICAL FLORA OF THE BRITISH ISLES* No. 283 List Vasc. PI. Br. Isles (1992) no. 153, 64,1 Biological Flora of the British Isles: Phragmites australis Jasmin G. Packer†,1,2,3, Laura A. Meyerson4, Hana Skalov a5, Petr Pysek 5,6,7 and Christoph Kueffer3,7 1Environment Institute, The University of Adelaide, Adelaide, SA 5005, Australia; 2School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia; 3Institute of Integrative Biology, Department of Environmental Systems Science, Swiss Federal Institute of Technology (ETH) Zurich, CH-8092, Zurich,€ Switzerland; 4University of Rhode Island, Natural Resources Science, Kingston, RI 02881, USA; 5Institute of Botany, Department of Invasion Ecology, The Czech Academy of Sciences, CZ-25243, Pruhonice, Czech Republic; 6Department of Ecology, Faculty of Science, Charles University, CZ-12844, Prague 2, Czech Republic; and 7Centre for Invasion Biology, Department of Botany and Zoology, Stellenbosch University, Matieland 7602, South Africa Summary 1. This account presents comprehensive information on the biology of Phragmites australis (Cav.) Trin. ex Steud. (P. communis Trin.; common reed) that is relevant to understanding its ecological char- acteristics and behaviour. The main topics are presented within the standard framework of the Biologi- cal Flora of the British Isles: distribution, habitat, communities, responses to biotic factors and to the abiotic environment, plant structure and physiology, phenology, floral and seed characters, herbivores and diseases, as well as history including invasive spread in other regions, and conservation. 2. Phragmites australis is a cosmopolitan species native to the British flora and widespread in lowland habitats throughout, from the Shetland archipelago to southern England. -

Micro Moths on Great Cumbrae Island (Vc100)
The Glasgow Naturalist (online 2017) Volume 26, xx-xx Micro moths on Great Cumbrae Island (vc100) P. G. Moore 32 Marine Parade, Millport, Isle of Cumbrae KA28 0EF E-mail: [email protected] ABSTRACT Forsythia sp. Behind the office is a large mature Few previous records exist for miCro-moths from black mulberry tree (Morus nigra) and to one side is vC100. Data are presented from the first year-round a tall privet hedge (Ligustrum ovalifolium). To the moth-trapping exerCise accomplished on Great rear of my property is a wooded escarpment with Cumbrae Island; one of the least studied of the old-growth ash (Fraxinus excelsior) frequently ivy- Clyde Isles (vC100). Data from a Skinner-type light- Covered (Hedera helix), sycamore (Acer trap, supplemented by Collection of leaf mines from pseudoplatanus) and rowan (Sorbus aucuparia), local trees, revealed the presence of 71 species of with an undergrowth of hawthorn (Crataegus miCro moths, representing 20 new records for the monogyna), wild garliC (Allium ursinum), nettle vice-County. (Urtica dioica), bracken (Pteridium aquilinum) and bramble (Rubus fructicosus). Rhind (1988) detailed INTRODUCTION the vasCular plants found on Great Cumbrae Island The extensive nineteenth-century list of between 1985 and 1987 and delineated the history Lepidoptera in the 1901 handbook on the natural of the island's botanical investigations. Leaves of history of Glasgow and the West of SCotland issued brambles in my garden, beech trees (Fagus for the Glasgow meeting of the British AssoCiation sylvatica) and hazel (Corylus avellana) at other for the Advancement of SCience (Elliot et al., 1901) locations on the island (respectively Craiglea Wood inCluded few Cumbrae records. -

Nuclear and Plastid DNA Phylogeny of the Tribe Cardueae (Compositae
1 Nuclear and plastid DNA phylogeny of the tribe Cardueae 2 (Compositae) with Hyb-Seq data: A new subtribal classification and a 3 temporal framework for the origin of the tribe and the subtribes 4 5 Sonia Herrando-Morairaa,*, Juan Antonio Callejab, Mercè Galbany-Casalsb, Núria Garcia-Jacasa, Jian- 6 Quan Liuc, Javier López-Alvaradob, Jordi López-Pujola, Jennifer R. Mandeld, Noemí Montes-Morenoa, 7 Cristina Roquetb,e, Llorenç Sáezb, Alexander Sennikovf, Alfonso Susannaa, Roser Vilatersanaa 8 9 a Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s.n., 08038 Barcelona, Spain 10 b Systematics and Evolution of Vascular Plants (UAB) – Associated Unit to CSIC, Departament de 11 Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de 12 Barcelona, ES-08193 Bellaterra, Spain 13 c Key Laboratory for Bio-Resources and Eco-Environment, College of Life Sciences, Sichuan University, 14 Chengdu, China 15 d Department of Biological Sciences, University of Memphis, Memphis, TN 38152, USA 16 e Univ. Grenoble Alpes, Univ. Savoie Mont Blanc, CNRS, LECA (Laboratoire d’Ecologie Alpine), FR- 17 38000 Grenoble, France 18 f Botanical Museum, Finnish Museum of Natural History, PO Box 7, FI-00014 University of Helsinki, 19 Finland; and Herbarium, Komarov Botanical Institute of Russian Academy of Sciences, Prof. Popov str. 20 2, 197376 St. Petersburg, Russia 21 22 *Corresponding author at: Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s. n., ES- 23 08038 Barcelona, Spain. E-mail address: [email protected] (S. Herrando-Moraira). 24 25 Abstract 26 Classification of the tribe Cardueae in natural subtribes has always been a challenge due to the lack of 27 support of some critical branches in previous phylogenies based on traditional Sanger markers.