Certolizumab Pegol for Rheumatoid Arthritis: Effective in Combination with Methotrexate Or As Monotherapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -

Old and New Challenges in Uveitis Associated with Behçet's Disease
Journal of Clinical Medicine Review Old and New Challenges in Uveitis Associated with Behçet’s Disease Julie Gueudry 1,* , Mathilde Leclercq 2, David Saadoun 3,4,5 and Bahram Bodaghi 6 1 Department of Ophthalmology, Hôpital Charles Nicolle, F-76000 Rouen, France 2 Department of Internal Medicine, Hôpital Charles Nicolle, F-76000 Rouen, France; [email protected] 3 Department of Internal Medicine and Clinical Immunology, AP-HP, Centre National de Références Maladies Autoimmunes et Systémiques Rares et Maladies Autoinflammatoires Rares, Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; [email protected] 4 Sorbonne Universités, UPMC Univ Paris 06, INSERM, UMR S 959, Immunology-Immunopathology-Immunotherapy (I3), F-75005 Paris, France 5 Biotherapy (CIC-BTi), Hôpital Pitié-Salpêtrière, AP-HP, F-75651 Paris, France 6 Department of Ophthalmology, IHU FOReSIGHT, Sorbonne-AP-HP, Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; [email protected] * Correspondence: [email protected]; Tel.: +33-2-32-88-80-57 Abstract: Behçet’s disease (BD) is a systemic vasculitis disease of unknown origin occurring in young people, which can be venous, arterial or both, classically occlusive. Ocular involvement is particularly frequent and severe; vascular occlusion secondary to retinal vasculitis may lead to rapid and severe loss of vision. Biologics have transformed the management of intraocular inflammation. However, the diagnosis of BD is still a major challenge. In the absence of a reliable biological marker, diagnosis is based on clinical diagnostic criteria and may be delayed after the appearance of the onset sign. However, therapeutic management of BD needs to be introduced early in order to control inflammation, to preserve visual function and to limit irreversible structural damage. -

Clinical Policy: Baricitinib (Olumiant) Reference Number: ERX.SPA.291 Effective Date: 07.24.18 Last Review Date: 11.18 Revision Log
Clinical Policy: Baricitinib (Olumiant) Reference Number: ERX.SPA.291 Effective Date: 07.24.18 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Baricitinib (Olumiant®) is Janus kinase (JAK) inhibitor. FDA Approved Indication(s) Olumiant is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more tumor necrosis factor (TNF) antagonist therapies. Limitation(s) of use: Use of Olumiant in combination with other JAK inhibitors, biologic disease-modifying antirheumatic drugs (DMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Envolve Pharmacy Solutions™ that Olumiant is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Rheumatoid Arthritis (must meet all): 1. Diagnosis of RA; 2. Prescribed by or in consultation with a rheumatologist; 3. Age ≥ 18 years; 4. Member meets one of the following (a or b): a. Failure of a ≥ 3 consecutive month trial of methotrexate (MTX) at up to maximally indicated doses, unless contraindicated or clinically significant adverse effects are experienced; b. If intolerance or contraindication to MTX (see Appendix D), failure of a ≥ 3 consecutive month trial of at least ONE conventional DMARD (e.g., sulfasalazine, leflunomide, hydroxychloroquine) at up to maximally indicated doses, unless contraindicated or clinically significant adverse effects are experienced; 5. -

Immunosuppressant Drugs Therapy with COVID-19
Immunosuppressant Drugs Therapy with COVID-19: Associated Risks, Drug-Drug Interactions & Contraindications Debjyoti Talukdar1, Diane Ignacio2, and Madan Mohan Gupta2 1Teerthanker Mahaveer University 2The University of the West Indies at St Augustine September 24, 2020 Abstract Immunosuppressant drugs like Etanercept, Mycophenolate mofetil, Sirolimus, Cyclosporine and Rituximab can weaken the immune system and make patients susceptible to SARS nCoV-2 virus. These drugs make immunocompromised persons more vulnerable to complications associated with COVID-19. Moreover, it can also increase the mortality and morbidity, as a weakened immune system can lead to longer duration of infection. This study discusses the guidelines on immunosuppressant drugs and its associated risk factors with COVID-19, issued by the U.S CDC (Centers for Disease Control and Prevention), WHO (World Health Organisation), U.S FDA (Food and Drug Administration) and other accredited global health organisa- tions. Moreover, it also includes information about pharmaceutical properties, mechanism of action, COVID-19 associated risk factors, adverse drug reactions, contraindications and drug-drug interactions. Our study will help government partners and international health organisations to better understand COVID-19 health risks associated with immunosuppressants. Increased public awareness about effective drug therapy for autoimmune diseases, cancer treatment, immunocompromised and organ transplant patients will help lower the mortality and morbidity associated with the disease amid COVID-19 pandemic. 1. INTRODUCTION As per the U.S Department of Health and Human Services and U.S FDA, immunosuppressant drugs can cause susceptibility to viruses leading to infection with increase in morbidity of the disease. It can cause severe risk of illness from COVID-19 due to a weakened immune system. -

Xolair/Omalizumab
Therapeutic/Humanized Antibodies ELISA Kits ‘Humanized antibodies” are ‘Animal Antibodies’ that have been modified by recombinant DNA-technology to reduce the overall content of the animal- portion of immunoglobulin so as to increase acceptance by humans or minimize ‘rejection’. By analogy, if the hands of a mouse is actually responsible for grabbing things then ‘humanized-mouse’ will only contain the ‘mouse hands’ and the rest of the body will be human. Since the size of the IgGs is similar in mouse and human, the size of the native mouse IgG and the ‘humanized IgG’ does not significantly change. Antigen recognition property of an antibody actually resides in small portion of the IgG molecule called the ‘antigen binding site or Fab’. Therefore, humanized antibodies contain the minimal portion from the Fab or the epitopes necessary for antigen binding. The process of "humanization of antibodies" is usually applied to animal (mouse) derived monoclonal antibodies for therapeutic use in humans (for example, antibodies developed as anti-cancer drugs). Xolair (Anti-IgE) is an example of humanized IgG. The portion of the mouse IgG that remains in the ‘humanized IgG’ may be recognized as foreign by humans and may result into the generation of “Human Anti-Drug Antibodies (HADA). The presence of anti-Drug antibody (e.g., Human Anti-Rituximab IgG) that may limit the long-term usage the humanized antibody (Rituximab). Not all monoclonal antibodies designed for human therapeutic use need be humanized since many therapies are short-term. The International Nonproprietary Names of humanized antibodies end in “-zumab”, as in “omalizumab”. Humanized antibodies are distinct from chimeric or fusion antibodies. -

Infliximab, Adalimumab and Golimumab for Treating Moderately
HEALTH TECHNOLOGY ASSESSMENT VOLUME 20 ISSUE 39 MAY 2016 ISSN 1366-5278 Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model Rachel Archer, Paul Tappenden, Shijie Ren, Marrissa Martyn-St James, Rebecca Harvey, Hasan Basarir, John Stevens, Christopher Carroll, Anna Cantrell, Alan Lobo and Sami Hoque DOI 10.3310/hta20390 Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model Rachel Archer,1* Paul Tappenden,1 Shijie Ren,1 Marrissa Martyn-St James,1 Rebecca Harvey,1 Hasan Basarir,1 John Stevens,1 Christopher Carroll,1 Anna Cantrell,1 Alan Lobo2 and Sami Hoque3 1Health Economics and Decision Science, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK 2Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK 3Barts Health NHS Trust, London, UK *Corresponding author Declared competing interests of authors: none Published May 2016 DOI: 10.3310/hta20390 This report should be referenced as follows: Archer R, Tappenden P, Ren S, Martyn-St James M, Harvey R, Basarir H, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model. Health Technol Assess 2016;20(39). Health Technology Assessment is indexed and abstracted in Index Medicus/MEDLINE, Excerpta Medica/EMBASE, Science Citation Index Expanded (SciSearch®) and Current Contents®/ Clinical Medicine. -

Benlysta® (Belimumab)
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2021 P 1227-6 Program Prior Authorization/Notification Medication Benlysta® (belimumab)* *This program applies to the subcutaneous formulation of belimumab P&T Approval Date 9/2017, 9/2018, 9/2019, 9/2020, 2/2021, 7/2021 Effective Date 10/1/2021; Oxford only: 10/1/2021 1. Background: Benlysta® is a B-lymphocyte stimulator (BLyS)-specific inhibitor indicated for the treatment of patients aged 5 years and older with active, autoantibody-positive, systemic lupus erythematosus (SLE) who are receiving standard therapy and adult patients with active lupus nephritis who are receiving standard therapy. Limitations of Use: The efficacy of Benlysta has not been evaluated in patients with severe active central nervous system lupus. Benlysta has not been studied in combination with other biologics. Use of Benlysta is not recommended in these situations. 2. Coverage Criteria: A. Systemic Lupus Erythematosus 1. Initial Authorization a. Benlysta will be approved based on all of the following criteria: (1) Diagnosis of systemic lupus erythematosus -AND- (2) Laboratory testing has documented the presence of autoantibodies [e.g., ANA, Anti-dsDNA, Anti-Sm, Anti-Ro/SSA, Anti-La/SSB] -AND- (3) Patient is currently receiving standard immunosuppressive therapy [e.g., hydroxychloroquine, chloroquine, prednisone, azathioprine, methotrexate] -AND- (4) Patient does not have severe active central nervous system lupus -AND- (5) Patient is not receiving Benlysta in combination with either of the following: (a) Biologic DMARD [e.g., Enbrel (etanercept), Humira (adalimumab), Cimzia (certolizumab), Kineret (anakinra)] © 2021 UnitedHealthcare Services, Inc. 1 (b) Lupkynis (voclosporin) Authorization will be issued for 12 months. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

Attachment: Extract from Clinical Evaluation: Etanercept
AusPAR Attachment 2 Extract from the Clinical Evaluation Report for etanercept Proprietary Product Name: Erelzi Sponsor: Novartis Pharmaceuticals Australia Pty Ltd First round report: June 2017 Second round report: August 2017 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) · The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. · The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. · The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. · The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. · To report a problem with a medicine or medical device, please see the information on the TGA website <https://www.tga.gov.au>. About AusPARs · An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. · AusPARs are prepared and published by the TGA. · An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. · An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. -

Heart Failure and Drug-Induced Lupus
Unusual toxicities with TNF inhibition: Heart failure and drug-induced lupus J.J. Cush John J. Cush, MD, Chief, Rheumatology ABSTRACT demyelinating disease, cytopenias, auto- and Clinical Immunology, Presbyterian Serious and unexpected adverse events, immune disorders, and heart failure. Hospital of Dallas, Clinical Professor of such as heart failure and drug-induced Other essays in this volume address the Internal Medicine, The University of Texas lupus, have been reported in patients subject of infection (10), demyelination Southwestern Medical School, Dallas, Texas, USA. receiving TNF inhibitor therapy. These (11), and lymphoma (12). This review events generally are easily recogniz- will focus on heart failure and lupus- Please address correspondence to: John J. Cush, MD, Presbyterian Hospital able, although they cannot be predicted like disease, which are rarely seen in of Dallas, 8200 Walnut Hill Lane, Dallas, nor avoided, other than by drug avoid- association with TNF inhibitor therapy Texax 75231-4496, USA. ance altogether. Many patients have and are currently without explanation. Clin Exp Rheumatol 2004; 22 (Suppl. 35): great benefit from anti-TNF therapies. S141-S147. Their intelligent use requires a firm Sources of data © Copyright CLINICAL AND EXPERIMENTAL understanding of these rare toxicities, As a consequence of drug approval, RHEUMATOLOGY 2004. so as to minimize the morbidity associ- manufacturers have a responsibility to ated with their uncommon occurrence. collect safety data actively and report Key words: TNF inhibitors, conges- these data to the appropriate agencies. tive heart failure, drug-induced lupus, Introduction For example, in the USA a manufactur- rheumatoid arthritis. The introduction of biologic agents to er must submit periodic safety reports specifically inhibit the pro-inflammato- to the FDA twice yearly (March and ry cytokine, tumor necrosis factor (TNF), September). -

New Biological Therapies: Introduction to the Basis of the Risk of Infection
New biological therapies: introduction to the basis of the risk of infection Mario FERNÁNDEZ RUIZ, MD, PhD Unit of Infectious Diseases Hospital Universitario “12 de Octubre”, Madrid ESCMIDInstituto de Investigación eLibraryHospital “12 de Octubre” (i+12) © by author Transparency Declaration Over the last 24 months I have received honoraria for talks on behalf of • Astellas Pharma • Gillead Sciences • Roche • Sanofi • Qiagen Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Paul Ehrlich (1854-1915) • “side-chain” theory (1897) • receptor-ligand concept (1900) • “magic bullet” theory • foundation for specific chemotherapy (1906) • Nobel Prize in Physiology and Medicine (1908) (together with Metchnikoff) Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author 1981: B-1 antibody (tositumomab) anti-CD20 monoclonal antibody 1997: FDA approval of rituximab for the treatment of relapsed or refractory CD20-positive NHL 2001: FDA approval of imatinib for the treatment of chronic myelogenous leukemia Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Functional classification of targeted (biological) agents • Agents targeting soluble immune effector molecules • Agents targeting cell surface receptors -
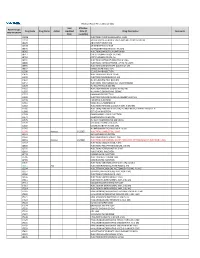
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB,