Abstract the Present Study Deals with the Diversity of Meiobenthic Fauna of Nathsagar Reservoir (Paithan) Dist
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Major Research Project Report of Chemistry
Major Research Project Report STUDIES ON INCIDENCE AND EXTENT OF PESTICIDE RESIDUE IN NATURAL WATER RESERVIORS IN WASHIM DISTRICT OF VIDARBHA REGION Submitted to UNIVERSITY GRANT COMMISSION BAHADURSHAH ZAFAR MARG, NEW DELHI – 110002 Submitted by Dr. N. S. THAKARE, Principal Investigator, M. S. P. Arts, Science and K. P. T. Commerce College, Manora Dist. Washim (M.S.) A CONSTITUENT COLLEGE UNDER S.G.B. AMRAVATI UNIVERSITY, AMRAVATI (MAHARASHTRA) CONTENTS Sr. Page No. Titles No. 1 Introduction 3-20 2 Objectives 21 3 Methods and Materials 22-23 4 Result 24-42 5 Discussion and Conclusion 43 6 References 44-46 Awareness of the peoples about the preventive and control 7 measure of pesticides residues in environment 47-49 2 Final Major Project Report From 01/04/2013 to 01/04/2016 Principal Investigator: - Dr. N. S. Thakare UGC File No.F.42-350/2013 Title: - “STUDIES ON INCIDENCE AND EXTENT OF PESTICIDE RECIDUES IN NATURAL WATER RESERVIORS IN WASHIM DISTRICT ON VIDARBHA REGION.” Amount Sanction: - 9, 33,000/- Introduction: - The term Pesticide is a composite term that includes all chemicals that are use to kill or control pest. Pesticide is a substance intended for preventing, destroying, repelling or migrating pests. A substance intended for use as plant growth regulator, defoliant or desiccant is also classified as pesticide. Pesticides are classified into insecticides, fungicides, herbicides or weedicides, acaricides, nematicides based on the target pest. The fundamental contribution to the green revolution has been the development and application of pesticides for the control of wide variety of insectivores and herbivores pests that would otherwise diminishes the quantity and quality of food products. -

Hingoli District, Maharashtra
1785/DBR/2013 भारत सरकार जल संसाधन मंत्रालय कᴂ द्रीय भूजल बो셍ड GOVERNMENT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD महाराष्ट्र रा煍य के अंतर्डत हहंर्ोली जजले की भूजल विज्ञान जानकारी GROUND WATER INFORMATION HINGOLI DISTRICT, MAHARASHTRA By 饍वारा S.D. WAGHMARE एस॰ 셍ी॰ िाघमारे Asst. Hydrogeologist सहायक भूजल िैज्ञाननक म鵍य क्षेत्र, नागपुर CENTRAL REGION, NAGPUR 2013 HINGOLI DISTRICT AT A GLANCE 1. GENERAL INFORMATION Geographical Area : 4827 sq. km. Administrative Divisions : Taluka-5; Hingoli, Sengaon, Aundha Nagnath, Kalamnuri and Vasmat. Villages : 710 Population (2001) : 986717 Average Annual Rainfall : 890.28 mm 2. GEOMORPHOLOGY Major Physiographic unit : Part of Western Ghats, Malhivra hill range, and Penganga plain Major Drainage : Penganga, Purna, Kayadu 3. LAND USE (2009-10) Forest Area : 275 sq. km. Net Area Sown : 4451.36 sq. km. Cultivable Area : 4509.42 sq. km. 4. SOIL TYPE : Black Cotton Soil 5. PRINCIPAL CROPS (2008-09) Cotton : 2545.00 sq. km. Cereals : 905.79 sq. km. Pulses : 9025.40 sq. km. Jowar : 3929.40 sq. km. Wheat : 2545.00 sq. km. 6. IRRIGATION BY DIFFERENT SOURCES (2000-01) - Nos. / Potential Created (ha)/ Potential Utilized(ha) Dugwells : 29049/75956/75924 Shallow Tubewells/ : 3000 / 8111 /8086 Deep Tubewells : 340 /1056 /1056 Surface Water : 7352 /23525 /23024 Net Irrigated Area : 108089 ha 7. GROUND WATER MONITORING WELLS (As on 31/05/2012) Dugwells : 42 Piezometers : Nil 8. GEOLOGY Recent : Alluvium Upper Cretaceous-Lower : Basalt (Deccan Traps) Eocene i 9. HYDROGEOLOGY Water Bearing Formation : Basalt (Deccan Traps) weathered, vesicular fractured, jointed. -

State: MAHARASHTRA Agriculture Contingency Plan for District
State: MAHARASHTRA Agriculture Contingency Plan for District: WASHIM 1.0 District Agriculture profile 1.1 Agro-Climatic/Ecological Zone DistrictAgro agricultureprofile Ecological Sub Region (ICAR) Eastern Maharashtra Plateau, hot moist semi-arid ESR with medium land deep clayey Black soils (shallow loamy to clayey Black soils as inclusion), medium to high AWC and LGP 120-150 days. (6.3) Agro-Climatic Zone (Planning Western plateau and hills region, Maharashtra (IX) Commission) Agro Climatic Zone (NARP) Central Vidarbha Zone (MH-8) List all the districts or part thereof Akola, Buldhana, Washim, Amravati falling under the NARP Zone Geographic coordinates of district Latitude Longitude Altitude headquarter : Washim 20° 05’58.90” N 77° 08'11.82” E 600M MSL Name and address of the concerned Agriculture Research Station, Washim-444805 ZRS/ ZARS/ RARS/ RRS/ RRTTS Mention the KVK located in the K.V.K. Karda Tq. Risod, Distt. Washim- 444805 district 1.2 Rainfall Normal RF(mm) Normal Rainy days Normal Onset Normal Cessation (number) SW monsoon (June-September): 848.6 41.3 2nd week of June 1st week of October NE Monsoon(October-December): 75.4 4.0 - - Winter (January-February) 26.7 2.1 Summer (March-May) 14.6 1.2 Annual 965.3 48.6 1 1.3 Land use Geographical Cultivable Forest Land Perman Cultivable Land under Barren Current Other pattern of the Area area area under non ent pastu waste miscellaneous & fallows fallows district (latest agricultur res land tree crops & unculti statistics) al use groves vable land Area (‘000 ha) 514 386 35 8 34 10 1 18 8 12 Source: * District Socio economic Review 2009 of respective district pub by Govt. -

Fact Sheets Fact Sheets
DistrictDistrict HIV/AIDSHIV/AIDS EpidemiologicalEpidemiological PrProfilesofiles developeddeveloped thrthroughough DataData TTriangulationriangulation FFACTACT SHEETSSHEETS MaharastraMaharastra National AIDS Control Organisation India’s voice against AIDS Ministry of Health & Family Welfare, Government of India 6th & 9th Floors, Chandralok Building, 36, Janpath, New Delhi - 110001 www.naco.gov.in VERSION 1.0 GOI/NACO/SIM/DEP/011214 Published with support of the Centers for Disease Control and Prevention under Cooperative Agreement No. 3U2GPS001955 implemented by FHI 360 District HIV/AIDS Epidemiological Profiles developed through Data Triangulation FACT SHEETS Maharashtra National AIDS Control Organisation India’s voice against AIDS Ministry of Health & Family Welfare, Government of India 6th & 9th Floors, Chandralok Building, 36, Janpath, New Delhi - 110001 www.naco.gov.in December 2014 Dr. Ashok Kumar, M.D. F.I.S.C.D & F.I.P.H.A Dy. Director General Tele : 91-11-23731956 Fax : 91-11-23731746 E-mail : [email protected] FOREWORD The national response to HIV/AIDS in India over the last decade has yielded encouraging outcomes in terms of prevention and control of HIV. However, in recent years, while declining HIV trends are evident at the national level as well as in most of the States, some low prevalence and vulnerable States have shown rising trends, warranting focused prevention efforts in specific areas. The National AIDS Control Programme (NACP) is strongly evidence-based and evidence-driven. Based on evidence from ‘Triangulation of Data’ from multiple sources and giving due weightage to vulnerability, the organizational structure of NACP has been decentralized to identified districts for priority attention. The programme has been successful in creating a robust database on HIV/AIDS through the HIV Sentinel Surveillance system, monthly programme reporting data and various research studies. -

BRIEF PROJECT REPORT Jan 2019
Consultancy Services for Project Management Phase I including Preparation of Detailed Project Report for up gradation of Nanded –Hingoli – Washim - Akola Section of NH-161 in the State of Maharashtra to Two/Four Lane with Paved Shoulder Configuration (Package No- NH/IAHE/05) Start Point End Point WarangaFata From Chaiange 4.635 (Akola) to Chaiange 92.200(upto Washim) of NH-161 BRIEF PROJECT REPORT Jan 2019 Consultants: Marc Technocrats Pvt. Ltd. in JV with Global Infra Solutions Marc House, Sector 6-7 (Dividing Road), Opp. Devi Lal Park, Bahadurgarh, Haryana-124507 Upgradation of Akola - Washim - Hingoli – Waranga Phata Section of NH-161) to Four Lane configuration in the state of Maharashtra on EPC mode Executive Summary of Section of Akola-Washim from Km 0.000 to Km 92.200 (Design Chainage) Project Report CONTENTS Executive Summary 1.1 General ................................................................................................................... 1 1.2 About the project .................................................................................................... 1 1.3 Necessity: ................................................................................................................ 1 1.3.1 Executive Summary ................................................................................................. 2 1.4 Project Description & Improvement Proposal .......................................................... 2 1.4.1 Existing Alignment .................................................................................................. -

Friday 13 March 2015 Issued By
Friday 13th March 2015 (For the period 13th to 17th March 2015) Issued by National Agrometeorological Advisory Service Centre, Agricultural Meteorology Division, India Meteorological Department, Shivajinagar, Pune. Standardised Precipitation Index Four Weekly th th for the Period 12 February to 11 March 2015 Extremely/severely wet conditions experienced in most districts of Himachal Pradesh, Gujarat State, Maharashtra & Goa, many districts of Uttarakhand, Haryana, Madhya Pradesh, Rajasthan, Karnataka, Telangana; few districts of Jammu and Kashmir, Punjab and Durg, Bijapur districts of Chhattisgarh; Upper Siang district of Arunachal Pradesh; Gopalganj, Khagaria districts of Bihar. Extremely /severely dry conditions experienced in East Kameng,West Kameng districts of Arunachal Pradesh. Moderately dry conditions experienced in Upper Subansiri district of Arunachal Pradesh; Cachar, Kokrajhar districts of Assam; Imphal East district of Manipur, Boudhgarh, Dhenkanal districts of Odisha; Seraikela district of Jharkhand; Raigarh district of Chhattisgarh. Rest of the country experienced moderately wet/ mild wet/dry conditions. Contour maps for Mean Maximum and Minimum Temperature and their anomaly for the week ending on 11.03.2015 Actual Mean Maximum Temperature (oC) in India for the Mean Maximum Temperature (oC) Anomaly in week ending 11.03.2015 India for the week ending 11.03.2015 28 to 360C over the country except Jammu & Kashmir, -6 to -40C isolated parts of Jammu & Kashmir, West Himachal Pradesh, Uttarakhand, Punjab, Haryana & Delhi, -

Maharashtra State Electricity Distibution Co. Ltd
T-06 Washim Maharashtra State Electricity Distibution Co. Ltd. Tender Details 22-04-2021 06:28:52 Tender Code T-06 Washim Tender Type Procurement Tender/Reverse Auction Type Of Bid Two Bid Description FOR PROCUREMENT OF 25 MW (AC) SOLAR POWER FROM PROJECTS TO BE DEVELOPED IN WASHIM DISTRICT OF MSEDCL THROUGH COMPETITIVE BIDDING PROCESS Estimated Cost (In Lakhs) 31834 Basis of prices Firm Price Basis Tender Validity 180 Delivery Requirement (In Months) Tender on rate contract basis NO Tender Fee (In INR) 25000 GST In INR (@18% on Tender Fee: SAC 4500 Total Tender Fee Amount including GST in INR. 29500 Mr Bhalchandra Gawai , 9920976517 Contact ,[email protected] Pre-Qualifying Req NA Budget Type NA Scheme Code NA Scheme Name MSKVY Department Renewable Energy Department Office Type HO Location Type Corporate Office Designation Executive Engineer(Distribution) Pre-Bid Meeting Address Prakashgad Bandra Bid Opening Address Prakashgad Bandra Version No 1 Call for Deviation NO Is Annexure C1 Applicable NO Is Manufacturer Applicable NO Is Trader Applicable NO Minimum % of Bid Quantum in MW 8 Is Power Supplier Applicable YES Tender Sale Start Date 22-04-2021 23:00 Tender Sale End Date 17-05-2021 10:00 Bid Start Date 22-04-2021 23:05 Bid End Date 17-05-2021 11:00 Pre-Bid Meeting Date 30-04-2021 12:00 Techno-Commercial Bid opening on 18-05-2021 14:00 Price Bid opening on Will be declared later Page 1 of 226 T-06 Washim Annexure C1 Opening Date 22-04-2021 18:19 Winner Selection Date Will be declared later Page 2 of 226 T-06 Washim Maharashtra State Electricity Distribution Co. -

Police Station Wise Polic Patil's of Washim District Sr
POLICE STATION WISE POLIC PATIL'S OF WASHIM DISTRICT SR. NAME OF NAME OF POLICE CONTACT NO. OF NAME OF VILLAGE NAME POLICE PATIL VILLAGE NO. THE UNIT STATION POLICE PATIL 1 WASHIM WASHIM RURAL ZAKALWADI ATMARAM UKANDA KALBANDE 9623765663 2 WASHIM WASHIM RURAL WAKAD KESHAV SHANKAR JADHAV 9765359597 3 WASHIM WASHIM RURAL PARDI TAKMOR SANTOSH DEVRAO CHAUDHARI 9922082918 4 WASHIM WASHIM RURAL SOMTHANA BALIRAM TUKARAM KHANDARE 7588884728 5 WASHIM WASHIM RURAL SUPKHELA VITHHAL SATVAJI THAKARE 9850374845 6 WASHIM WASHIM RURAL KAKADADATI GANPAT NIVRUTTI SAKHARKAR 9921020796 7 WASHIM WASHIM RURAL JANUNA ATAMRAM SAVAIRAM RATHOD 9552036379 8 WASHIM WASHIM RURAL PANDAV UMARA SUBHASH NAGOJI DHOBALE 9623737630 9 WASHIM WASHIM RURAL TANDAI SHEVAI DEVRAO NIVRUTTI BHALERAO 9850510960 10 WASHIM WASHIM RURAL WAGHADARI ASHOK RAMBHAU WANKHADE 8550962683 11 WASHIM WASHIM RURAL KONDALA MAHALI KUNDALIK HARISING RATHOD 9673860044 12 WASHIM WASHIM RURAL KUMBHARKHEDA NAMDEV GOVINDA THAKARE 7588090345 13 WASHIM WASHIM RURAL SAVANGA JAHA. PRALHAD TULSHIRAM MOGHAD 8806852256 14 WASHIM WASHIM RURAL KEKATUMARA DATTA SAKHARAM WANKHEDE 9623763662 15 WASHIM WASHIM RURAL EKBURUJI NIVRUTTI GOVINDA SARKATE 9822590853 16 WASHIM WASHIM RURAL BORKHEDI PRABHAKAR TUKARAM KALBANDE 9763201980 17 WASHIM WASHIM RURAL GANESHPUR SAHEBRAO PARASHRAM WAGHAMARE 9623837058 18 WASHIM WASHIM RURAL WAGHULI BU. WAMAN RAJARAM SHINDE 9673151494 19 WASHIM WASHIM RURAL JUMDA BHANUDAS YADAV PADGHAN 9011750030 20 WASHIM WASHIM RURAL WAGHOLI KHU. EKNATH SHAHAJI WAKUDAKAR 9158354510 21 WASHIM WASHIM -

Handbook on Fisheries Statistics 2018 2018
HANDBOOK ON FISHERIES STATISTICS HANDBOOK ON FISHERIES STATISTICS 2018 HANDBOOK ON FISHERIES STATISTICS 2018 Government of India Ministry of Fisheries, Animal Husbandry and Dairying Department of Fisheries Krishi Bhavan, New Delhi Fish Production During recent Years 140.00 125.90 120.00 114.31 107.62 102.60 95.79 90.40 100.00 89.02 86.66 82.31 79.98 78.06 76.16 71.62 71.27 80.00 68.69 66.91 65.72 61.36 57.19 52.94 49.81 60.00 48.94 46.38 42.07 38.45 37.56 36.88 36.25 36.00 35.69 34.43 33.72 33.21 32.50 31.04 30.24 29.78 (In Lakh Lakh tonnes) (In 29.20 40.00 28.16 20.00 0.00 2005-06 2006-07 2007-08 2008-09 2009-10 2010-11 2011-12 2012-13 2013-14 2014-15 2015-16 2016-17 2017-18 year Marine Inland Total HANDBOOK ON FISHERIES STATISTICS 2018 September 2019 Department of Fisheries Ministry of Fisheries, Animal Husbandry & Dairying Govt. of India, New Delhi Compiled by Fisheries Statistics Division Department of Fisheries Ministry of Fisheries, Animal Husbandry & Dairying Government of India Printed by Fishery Survey of India on behalf of Department of Fisheries Printed at Onlooker Press, 16, Sassoon Dock, Mumbai - 400 005. Tel.: +91 22 2218 2939/ 3544, Email : [email protected] CONTENTS TABLES Page SECTION A : PRODUCTION AND DISPOSAL A - 1: Fish Production in India for the Period - 1950-51 to 2017- 18 5 A - 2: State/UT wise Inland and Marine Fish Production 2011-12 to 2017-18 8 A - 3: State / Union Territory wise Fish Production 2011-12 to 2017-18 11 A - 4: State / Union Territory wise Inland Fish Production 2011-12 to 2017-18 12 A - -

National Wetland Atlas: Maharashtra
NATIONAL WETLAND ATLAS: MAHARASHTRA Sponsored by Ministry of Environment and Forests Government of India Space Applications centre Indian Space Research Organisation Ahmedabad – 380 015 This publication deals with the updated database and status of wetlands, compiled in Atlas format. Increasing concern about how our wetlands are being influenced has led to formulation of a project entitled “National Wetland Inventory and Assessment (NWIA)” to create an updated database of the wetlands of India. The wetlands are categorised under 19 classes and mapped using satellite remote sensing data from Indian Remote Sensing Satellite: IRS P6- LISS III sensor. The results are organised at 1: 50, 000 scales at district, state and topographic map sheet (Survey of India reference) level using Geographic Information System (GIS). This publication is a part of this national work and deals with the wetland status of a particular State/Union Territory of India, through text, statistical tables, satellite images, maps and ground photographs. The atlas comprises wetland information arranged into nine sections. How the NWIA project work has been executed highlighted in the first six sections viz: Introduction, NWIA project, Study area, Data used, Methodology, and Accuracy. This is the first time that high resolution digital remote sensing data has been used to map and decipher the status of the wetlands at national scale. The methodology highlights how the four spectral bands of LISS III data (green, red, near infra red and short wave infra red) have been used to derive various indices and decipher information regarding water spread, turbidity and aquatic vegetation. Since, the aim was to generate a GIS compatible database, details of the standards of database are also highlighted in the methodology. -
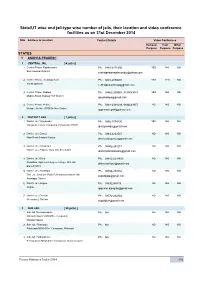
State/UT Wise and Jail-Type Wise Number of Jails, Their Location and Video Conference Facilities As on 31St December 2014
State/UT wise and jail-type wise number of jails, their location and video conference facilities as on 31st December 2014 SNo Address & Location Contact Details Video Conference Remand Trial Other Purpose Purpose Purpose STATES 1 ANDHRA PRADESH 1 CENTRAL JAIL [ 4 jail(s) ] 1 Central Prison, Rajahmundry Ph.: 0883-2471990 YES NO NO East Godavari District [email protected] 2 Central Prison, Visakhapatnam Ph.: 0891-2870601 YES YES NO Visakhapatnam [email protected] 3 Central Prison, Kadapa Ph.: 08562-200559, 9494633643 YES NO NO Madras Road, Kadapa YSR District [email protected] 4 Central Prison, Nellore Ph.: 0861-2331639, 9494633857 NO NO NO Mulapet, Nellore SPSR Nellore District [email protected] 2 DISTRICT JAIL [ 7 jail(s) ] 1 District Jail, Vijayawada Ph.: 0866-2574236 YES NO NO Vijaywada Taluka Compound Vijayawada-520003 [email protected] 2 District Jail, Guntur Ph.: 0863-2232547 NO NO NO Main Road Brodipet Guntur [email protected] 3 District Jail, Srikakulam Ph.: 08942-241251 NO NO NO District Jail, Ampolu, Gara (M), Srikakulam [email protected] 4 District Jail, Eluru Ph.: 08812-2244633 NO NO NO Kotadibba, Opp Govt. degree College, Dist Jail, [email protected] Eluru-534001 5 District Jail, Anantapur Ph.: 08554-257232 NO NO NO Dist. Jail Jantaloor (Post) Bukkarayasamudram (M) [email protected] Anantapur District 6 District Jail, Ongole Ph.: 08592280419 NO NO NO Ongole [email protected] 7 District Jail, Chittoor Ph.: 08572-242844 NO NO NO Greamspet, -

A Baseline Survey of Minority Concentration Districts of India Washim
A BASELINE SURVEY OF MINORITY CONCENTRATION DISTRICTS OF INDIA WASHIM (Maharashtra) Sponsored by Ministry of Minority Affairs Government of India and Indian Council of Social Science Research INSTITUTE FOR HUMAN DEVELOPMENT NIDM Building, 3rd Floor, IIPA Campus I.P. Estate, Mahatma Gandhi Marg, New Delhi-110 002 Phones – 2335 8166, 2332 1610 / Fax : 23765410 Email: [email protected], website:ihdindia.org 2008 A BASELINE SURVEY OF MINORITY CONCENTRATION DISTRICTS OF INDIA WASHIM (Maharashtra) Sponsored by Ministry of Minority Affairs Government of India and Indian Council of Social Science Research INSTITUTE FOR HUMAN DEVELOPMENT NIDM Building, 3rd Floor, IIPA Campus I.P Estate, Mahatma Gandhi Marg, New Delhi-110 002 Phones – 2335 8166, 2332 1610 / Fax : 23765410 Email: [email protected], website:ihdindia.org RESEARCH TEAM Principal Researchers Alakh N. Sharma Ashok K. Pankaj Data Processing and Tabulation Balwant Singh Mehta Sunil Kumar Mishra Abhay Kumar Research Associates/Field Supervisors Ramashray Singh Ashwani Kumar Subodh Kumar M. Poornima Research Assistant P.K. Mishra Secretarial Assistance Shri Prakash Sharma Nidhi Sharma Sindhu Joshi WASHIM Principal Author of the Report Reshmi P. Bhaskaran Fellow Institute for Human Development CONTENTS Executive Summary....................................................................................................i-viii CHAPTER I: INTRODUCTION .....................................................................................1-7 Methodology.....................................................................................................................