Transport Constraints on Water Use by the Great Basin Shrub, Artemisia Tridentata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CDFG Natural Communities List
Department of Fish and Game Biogeographic Data Branch The Vegetation Classification and Mapping Program List of California Terrestrial Natural Communities Recognized by The California Natural Diversity Database September 2003 Edition Introduction: This document supersedes all other lists of terrestrial natural communities developed by the Natural Diversity Database (CNDDB). It is based on the classification put forth in “A Manual of California Vegetation” (Sawyer and Keeler-Wolf 1995 and upcoming new edition). However, it is structured to be compatible with previous CNDDB lists (e.g., Holland 1986). For those familiar with the Holland numerical coding system you will see a general similarity in the upper levels of the hierarchy. You will also see a greater detail at the lower levels of the hierarchy. The numbering system has been modified to incorporate this richer detail. Decimal points have been added to separate major groupings and two additional digits have been added to encompass the finest hierarchal detail. One of the objectives of the Manual of California Vegetation (MCV) was to apply a uniform hierarchical structure to the State’s vegetation types. Quantifiable classification rules were established to define the major floristic groups, called alliances and associations in the National Vegetation Classification (Grossman et al. 1998). In this document, the alliance level is denoted in the center triplet of the coding system and the associations in the right hand pair of numbers to the left of the final decimal. The numbers of the alliance in the center triplet attempt to denote relationships in floristic similarity. For example, the Chamise-Eastwood Manzanita alliance (37.106.00) is more closely related to the Chamise- Cupleaf Ceanothus alliance (37.105.00) than it is to the Chaparral Whitethorn alliance (37.205.00). -

Facilitation of Yucca Brevifolia Recruitment by Mojave Desert Shrubs
UNLV Retrospective Theses & Dissertations 1-1-1998 Facilitation of Yucca brevifolia recruitment by Mojave Desert shrubs Steve B Brittingham University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/rtds Repository Citation Brittingham, Steve B, "Facilitation of Yucca brevifolia recruitment by Mojave Desert shrubs" (1998). UNLV Retrospective Theses & Dissertations. 950. http://dx.doi.org/10.25669/ms22-zauw This Thesis is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in UNLV Retrospective Theses & Dissertations by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter free, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

Sagebrush Bibliography
For Research Use Rio Grande National Forest – Sagebrush Bibliography Prepared by Janine Rice, PhD Rice Consulting LLC November 10, 2016 Contents Overview ......................................................................................................................................... 1 Sagebrush: Ecology and Climate .................................................................................................... 3 Sagebrush: Carbon Cycle and Climate ........................................................................................... 7 Sagebrush: Fire, Stressors and Climate .......................................................................................... 8 Sagebrush: Vegetation Modeling .................................................................................................... 9 Sagebrush: Management and Restoration ..................................................................................... 14 Relevant Publications in other Bibliography Chapters ................................................................. 17 Vulnerability Assessments and Reports Bibliography ............................................................. 17 Spruce-Fir Bibliography ........................................................................................................... 17 --Ctrl/Click to follow link above Overview This bibliography provides relevant scientific citations with abstracts on topics identified by the Rio Grande National Forest staff as important to the development of their assessment and plan -

Sagebrush Identification Guide
Sagebrush Identification Table For Use With Black Light For Use in the Inter-Great Basin Area Fluoresces Under Ultraviolet Branching Mature Plant Plant Nomenclature Light Leaf shape and size Plant Growth Form Environment Comments Pattern Height Water Alcohol Leaves 3/4 ‐1 1/4 in. Uneven topped; Main stem is undivided and trunk‐like at base;. Located long; long narrow; Leaf Uneven normally in drainage bottoms; Small concave areas and valley floors, but will normally be 4 times Colorless to Very topped; always on deep Non‐saline Non‐calcareous soils. Vegetative leader is greater Brownish to longer than it is at its "V"ed Mesic to Frigid 3.5 ft. to Very Pale blue Floral stems than 1/2 the length of the flower stalk from the same single branch. In Basin Basin Big Sagebrush Artemisia Reddish‐Brown widest point; Leaf branching/ Xeric to Ustic greater than 8 tridentata subsp. tridentata (ARTRT) Rarely pale growing there are two growth forms: One the Typical tall form (Diploid); Two a shorter to colorless margins not extending upright 4000 to 8000 ft. ft. Brownish‐red throughout form that looks similar to Wyoming sagebrush if you do not look for the trunk outward; Crushed leaves the crown (around 1 inch or so); the branching pattern; and the seedhead to vegetative have a strong turpentine leader characteristics (Tetraploid). smell Uneven Leaves 1/2 ‐ 3/4 inches topped; Uneven topped; Main stem is usually divided at ground level. Plants will often Mesic to Frigid Wyoming Big Sagebrush Colorless to Very Colorless to pale long; Leaf margins curved Floral stems Spreading/ keep the last years seed stalks into the following fall. -

Common Shrubs Shrub-Steppe Habitats
Common shrubs shrub-steppe habitats Photos (unless noted) by Susan Ballinger Sources for text include: http://biology.burke.washington.edu/herbarium/imagecollection.php Flora of the Pacific Northwest by C. Leo Hitchcock & Arthur Cronquist Plants of Southern Interior British Columbia and the Inland Northwest by Roberta Parish, Ray Coupe, and Dennis Lloyd Fall, 2012 Artemisia tridentata big sagebrush ASTER Family Habitat: widespread and common in deep soiled (>12 in.) shrub-steppe up to 7 feet tall Prior fall’s flowering stalks. Leaves: wedge-shaped, most with 3 toothed-tip. Dense gray hair on both sides. Most leaves persist through winter. Yellow in photo are long thin leaves, that dry up & die in summer. Smaller hairy, thick leaves remain year-round Flowers: small, yellow, born in composite Flowers in fall. Does not re- heads of 3-5 sprout after wildfire but disk flowers. Evergreen aromatic shrub. Grayish regenerates from seed. Very small. shredding bark on older branches. Artemisia tripartita three-tip sagebrush ASTER Family Habitat: Generally smaller shrub than big sagebrush, growing in slightly moister sites. Leaves: deeply cleft into narrow linear divisions, 2-4 ft. tall which may themselves be 3-cleft A. tridentata A. tripartita Flowers in fall, evergreen 1-2 feet tall. Vigorous sprouter after wildfire. Flower buds appear brown Artemesia rigida rigid sagebrush ASTER Family habitat: dry, rocky, thin soils in shrub-steppe. Less than 2 feet tall Small, often spreading outward on ground. Older bark is very black. Flowers in fall Leaves: 1-4 cm. long, narrow, deeply divided into 3-5 narrow segments. All deciduous leaves Flowers: heads or clusters of heads sessile in the axils, surrounded by longer leaves. -

Known High Quality Or Rare Plant Communities and Wetland Ecosystems
Appendix A Upper Middle Mainstem Columbia River Subbasin Plan Known High Quality or Rare Plant Communities and Wetland Ecosystems Table 1 Known high quality or rare plant communitites and wetland ecosystems of the UMM Subbasin, WA. SCIENTIFIC NAME COMMON NAME Abies amabilis - Tsuga mertensiana cover Pacific silver fir - mountain hemlock type forest Abies amabilis / Achlys triphylla forest Pacific silver fir / vanillaleaf Abies amabilis cover type Pacific silver fir forest Abies grandis / Acer circinatum forest Grand fir / vine maple Abies lasiocarpa / Calamagrostis rubescens Subalpine fir / pinegrass forest Abies lasiocarpa / Ledum glandulosum forest Subalpine fir / glandular labrador-tea Abies lasiocarpa / Rhododendron albiflorum Subalpine fir / cascade azalea woodland Abies lasiocarpa /Vaccinium scoparium Subalpine fir / grouseberry forest Abies lasiocarpa cover type Subalpine fir forest Abies procera cover type Noble fir forest Acer circinatum cover type Vine maple shrubland Alnus viridis ssp. Sinuata shrubland Sitka alder (provisional) SCIENTIFIC NAME COMMON NAME Artemisia arbuscula / Festuca idahoensis Low sagebrush /Idaho fescue dwarf-shrub herbaceous vegetation Artemisia rigida / Poa secunda dwarf-shrub Stiff sagebrush / Sandberg's bluegrass herbaceous vegetation Artemisia rigida cover type Stiff sagebrush shrubland Artemisia tridentata / Festuca idahoensis Big sagebrush / Idaho fescue shrub herbaceous vegetation Artemisia tridentata cover type Big sagebrush shrubland Artemisia tridentata ssp. Wyomingensis / Wyoming big sagebrush -
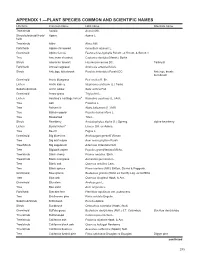
APPENDIX 1.—PLANT SPECIES COMMON and SCIENTIFIC NAMES Life Form Common Name Latin Name Alternate Name Tree/Shrub Acacia Acacia Mill
APPENDIX 1.—PLANT SPECIES COMMON AND SCIENTIFIC NAMES Life form Common name Latin name Alternate name Tree/shrub Acacia Acacia Mill. Shrub/subshrub//Forb/ Agave Agave L. herb Tree/shrub Alder Alnus Mill. Forb/herb Alpine chickweed Cerastium alpinum L. Graminoid Alpine fescue Festuca brachyphylla Schult. ex Schult. & Schult. f. Tree American chestnut Castanea dentata (Marsh.) Borkh. Shrub American tarwort Flourensia cernua DC. Tarbrush Forb/herb Annual ragweed Ambrosia artemisiifolia L. Shrub Antelope bitterbrush Purshia tridentata (Pursh) DC. Antelope brush; buckbrush Graminoid Arctic bluegrass Poa arctica R. Br. Lichen Arctic kidney Nephroma arcticum (L.) Torss. Subshrub/shrub Arctic willow Salix arctica Pall. Graminoid Arrow grass Triglochin L. Lichen Asahina’s cartilage lichend Ramalina asahinae (L.) Ach. Tree Ash Fraxinus L. Tree Balsam fi r Abies balsamea (L.) Mill. Tree Balsam poplar Populus balsamifera L. Tree Basswood Tilia L. Shrub Bearberry Arctostaphylos alpina (L.) Spreng. alpine bearberry Lichen Beard lichena Usnea Dill. ex Adans. Tree Beech Fagus L. Graminoid Big bluestem Andropogon gerardii Vitman Tree Big leaf maple Acer macrophyllum Pursh Tree/Shrub Big sagebrush Artemisia tridentata Nutt. Tree Bigtooth aspen Populus grandidentata Michx. Tree/shrub Black cherry Prunus serotina, Ehrh. Tree/shrub Black mangrove Avicennia germinans L. Tree Black oak Quercus velutina, Lam. Tree Black spruce Picea mariana (Mill.) Britton, Sterns & Poggenb. Graminoid Blue grama Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffi ths Tree Blue oak Quercus douglasii Hook. & Arn. Graminoid Bluestem Andropogon L. Tree Box elder Acer negundo L. Forb/herb Bracken fern Pteridium aquilinum var. pubescens Tree Bristlecone pine Pinus aristata Engelm. Subshrub/Shrub Brittlebush Encelia Adans. -

Project Budburst Available Species Sheet
Project BudBurst Available Species Sheet www.budburst.org Wildflowers and Herbs Deciduous Trees and Shrubs • Alfalfa (Medicago sativa) • American linden (Tilia americana) • American pasqueflower (Pulsatilla patens aka • Antelope bitterbrush (Purshia tridentata) Anemone patens) • Apple (Malus pumila) • Bigleaf lupine (Lupinus polyphyllus) • Bald cypress (Taxodium distichum) • Bitter root (Lewisia rediviva) • Balsam poplar (Populus balsamifera (aka • California poppy (Eschscholzia californica) trichocarpa)) • Canada thistle (Cirsium arvense) • Beaked hazelnut (Corylus cornuta) • Colorado blue columbine (Aquilegia caerulea) • Bigleaf maple (Acer macrophyllum) • Common dandelion (Taraxacum officinale) • Black elderberry (Sambucus nigra) • Common yarrow (Achillea millefolium) • Black locust (Robinia pseudoacacia) • Darkthroat shootingstar (Dodecatheon • Boxelder (Acer negundo) pulchellum) • Chokecherry (Prunus virginiana) • Dogtooth violet (Erythronium americanum) • Common lilac (Syringa vulgaris) • Field mustard (Brassica rapa) • Common snowberry (Symphoricarpos albus) • Henbit deadnettle (Lamium amplexicaule) • Eastern serviceberry (Amelanchier canadensis) • Indian pink (Spigelia marilandica) • Flowering dogwood (Cornus florida) • Jack in the pulpit (Arisaema triphyllum) • Forsythia (Forsythia xintermedia) • Lanceleaf springbeauty (Claytonia lanceolata) • Lewis' mock orange (Philadelphus lewisii) • Large flowered trillium (Trillium grandiflorum) • Pacific dogwood (Cornus nuttallii) • Mayapple (Podophyllum peltatum) • Paper birch (Betula -

Successfully Storing Milkweed Taproots for Habitat Restoration
Asclepias speciosa, or showy milkweed, is the most common milkweed species in the Great Plains and western US. Photo by Tom Koerner, US F&WS 48 NATIVEPLANTS | 20 | 1 | SPRING 2019 REFEREED RESEARCH Successfully storing milkweed taproots for habitat restoration Melissa L Topping, R Kasten Dumroese, and Jeremiah R Pinto ABSTRACT During a 3-y seed increase project, taproot size (volume and biomass) of showy milkweed (Asclepias speciosa Torr. [Asclepiadaceae]) increased annually, although top diameter of the taproot remained constant after the first growing season. At the end of the third year, taproots were harvested, held under different cold storage treatments, and subsequently outplanted. We observed that taproots can readily be stored in cardboard boxes placed in either cooler (0.5 to 2 °C [33–36 °F]) or freezer (–5 to –2 °C [23–28 °F]) conditions, and that success increases with the addition of protection from the cold in the form of plastic bag liners and (or) peat moss; survival was as high as 90%. These findings are promising because milkweeds are essential to the life cycle of the monarch butterfly Danaus( plexip pus L. [Lepidoptera: Nymphalidae]), which has experienced large population declines dur- ing the past 20 y due primarily to habitat loss and fragmentation. Restoring milkweed to the environment is an important first step to help bolster populations. Seed increase is often used to produce seeds for specific restoration projects, but the taproots are often discarded once sufficient seeds have been attained. Harvesting the taproots is an effective way to efficiently use all parts of the increase bed. -

Wild. Local. Beautiful. Center for Native Plants Most Deer Resistant
Wild. Local. Beautiful. Center for Native Plants Most Deer Resistant Species Keep in mind that the only deer-proof plant is one enclosed by a fence. The list of plants below are known to be deer resistant, but deer will eat any plant when hungry enough, say in the spring or during a drought, and fawn will sample anything as they learn what’s good to eat. FORBS Yarrow (Achillea millefolium) Alumroot (Heuchera cylindrica) Yellow Prairie Coneflower (Ratibida columnifera) Arrowleaf Balsamroot (Balsamorhiza sagittata) Yucca (Yucca glauca) Aspen Fleabane (Erigeron speciosus) Beebalm (Monarda fistulosa) SHRUBS Black-eyed Susan (Rudbeckia hirta) Big Sage (Artemisia tridentata) Blanketflower (Gaillardia aristata) Black Hawthorn (Crataegus douglasii) Canada Goldenrod (Solidago canadensis Buffaloberry (Shepherdia canadensis) Cutleaf Daisy (Erigeron compositus) Fernbush (Chamaebatiaria millefolium) Dotted Blazing Star (Liatris punctata) Juniper spp. (Juniperus spp.) Fuzzy-tongue Penstemon (Penstemon eriantherus) Rubber Rabbitbrush (Ericameria nauseosa) Hairy Golden Aster (Heterotheca villosa) Serviceberry (Amelanchier alnifolia) Heart-leaf Arnica (Arnica cordifolia) Shrubby Cinquefoil (Dasiphora fruticosa) Horsemint (Agastache urticifolia) Three-leaved Sumac (Rhus trilobata) Lanceleaf Daisy (Erigeron linearis) Wax Currant (Ribes cereum) Leafy Aster (Symphyotrichum foliaceum) White Sage (Artemisia ludoviciana) Munro’s Globemallow (Sphaeralcea munroana) GRASSES Nodding Onion (Allium cernuum) Most of our grasses are deer resistant. Ask our native Pearly Everlasting (Anaphalis margaritacea) plant specialists about which ones aren’t. Plains Coreopsis (Coreopsis tinctoria) Prairie Smoke (Geum triflorum) GROUNDCOVERS Prickly Pear Cactus (Opuntia fragilis) Birch-leaved Spirea (Spiraea betulifolia) Purple Coneflower (Echinacea angustifolia) Kinnikinnick (Arctostaphylos uva-ursi) Rocky Mountain Beeplant (Peritoma serrulata) Oregon-grape (Berberis repens) Rosy Pussytoes (Antennaria rosea) Showy Goldeneye (Heliomerus multiflora) TREES Showy Milkweed (Asclepias speciosa) Betula spp. -

Contents STBL
Contents STBL ........................................................................................................................................................... 1 Species ...................................................................................................................................................... 1 Species by Module .................................................................................................................................... 3 STBL STBL is the species to be learned for each module. Each module will have 10 species to be identified. Through identification students will learn plant physiology and adaptations. This will be the essential plant species students will need to know to successfully understand the EBIPM program. It is recommended that the class consistently uses one or more of the following techniques as a learning activity or understanding species. Wanted posters Herbarium (template) ID card (template) Resources NRCS Plant guides. Reynolds Creek Watershed Plant Guide. By ARS & NRCS Grasses identification Species Trees Western Juniper- JUOC-Juniperus occidentalis Utah Juniper- JUOS-Juniperus osteosperma Douglas Fir- PSMEM-Pseudotsuga menziesii Ponderosa Pine-PIPO-Pinus ponderosa Quaking Aspen-POTR5-Populus tremuloides Shrubs Big Sagebrush-ARTR2-Artemisia tridentata Wyoming Big Sagebrush-ARTRW8-Artemisia tridentata Nutt. ssp. wyomingensis Basin Big Sagebrush-ARTRT-Artemisia tridentata Nutt. ssp. tridentata Mountain Big Sagebrush-ARTRV-Artemisia tridentata Nutt. ssp. vaseyana -

2020 Plant & Seed Brochure
NATIVE GRASSES PLANTS are grown from Alberta-collected seed, Achnatherum richardonii- Richardson’s Needle Grass started in the greenhouse, and grown mainly in plug Bouteloua gracilis – Blue Grama containers. 2020 Plant & Seed Brochure Bromus ciliatus – Fringed Brome Danthonia parryi – Parry Oatgrass SEED is available for many species. If we don’t have Deschampsia caespitosa – Tufted Hairgrass what you need we could source it for you. Elymus canadensis – Canada Wild Rye Elymus innovatus – Hairy Wild Rye CONSULTING is provided in plant selection, design, Festuca campestris – Foothills Rough Fescue and planting/seeding your project area. We can also Festuca saximontana – Rocky Mountain Fescue help you manage or maintain your site. Hierachloe odorata – Sweetgrass Koeleria macrantha – Junegrass Please contact us regarding species, potential Nassella viridula – Green Needle Grass mixes, and pricing. Pascopyrum smithii – Western Wheatgrass Poa alpinum – Alpine Bluegrass PRICES Schizachyrium scoparium – Little Bluestem Individual Seed Packets $3.50 each Trisetum spicatum – Spike Trisetum Wildflower Seed Mix Packets $5.00 each NATIVE SHRUBS Plant plugs $4.00 each Paintbrush plugs $4.50 each Amelanchier alnifolia – Saskatoon Berry Shooting star plugs $4.50 each Arctostaphylos uva- ursi – Bearberry All prices are subject to change depending on species, Artemisia cana – Silver Sagebrush availability and production costs. Artemisia frigida – Fringed Sage (pasture) Artemisia ludoviciana – Prairie Sage Artemisia tridentata – Big Sagebrush Minimum handling