19660018236.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Light Isotopes of Berkelium and Californium
Lawrence Berkeley National Laboratory Recent Work Title LIGHT ISOTOPES OF BERKELIUM AND CALIFORNIUM Permalink https://escholarship.org/uc/item/1fm7b1fp Author Chetham-Strode, Alfred Publication Date 1956-06-26 eScholarship.org Powered by the California Digital Library University of California .... I UCRL 3322 UNIVERSITY OF CALIF-ORNIA .. i/ I / LIGHT ISOTOPES OF BERKELIUM AND CALIFORNIUM •' TWO-WEEK LOAN COPY This is a Library Circulating Copy r 'which may be borrowed for two weeks. For a personal retention copy, call Tech. Info. Dioision, Ext. 5545 DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of · California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. UCRL- 3322 Chemistry Distribution UNIVERSITY OF CALIFORNIA Radiation Laboratory Berkeley, California Contract No. W-7405-eng-48 LIGHT ISOTOPES OF BERKELIUM AND CALI~ORNIUM Alfred Chetham-Strode, Jr. -

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements . -

1. Public Health Statement
AMERICIUM 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about americium and the effects of exposure. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for long-term federal cleanup activities. Americium has been found in at least 8 of the 1,636 current or former NPL sites. However, the total number of NPL sites evaluated for americium is not known. As more sites are evaluated, the sites at which americium is found may increase. This information is important because exposure to americium may harm you and because these sites may be sources of exposure. When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. You are normally exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. However, since americium is radioactive, you can also be exposed to its radiation if you are near it. External or internal exposure to radiation may occur from natural or man-made sources. Naturally occurring sources of radiation are cosmic radiation from space or naturally occurring radioactive materials in our body or in soil, air, water, or building materials. Man-made sources of radiation are found in consumer products, industrial equipment, atom bomb fallout, and to a smaller extent, from hospital waste and nuclear reactors. -

Properties of Selected Radioisotopes
CASE FILE COPY NASA SP-7031 Properties of Selected Radioisotopes A Bibliography PART I: UNCLASSIFIED LITERATURE NATIONAL AERONAUTICS AND SPACE ADMINISTRATION NASA SP-7031 PROPERTIES OF SELECTED RADIOISOTOPES A Bibliography Part I: Unclassified Literature A selection of annotated references to technical papers, journal articles, and books This bibliography was compiled and edited by DALE HARRIS and JOSEPH EPSTEIN Goddard Space Flight Center Greenbelt, Maryland Scientific and Technical Information Division / OFFICE OF TECHNOLOGY UTILIZATION 1968 USP. NATIONAL AERONAUTICS AND SPACE ADMINISTRATION Washington, D.C. PREFACE The increasing interest in the application of substantial quantities of radioisotopes for propulsion, energy conversion, and various other thermal concepts emphasizes a need for the most recent and most accurate information available describing the nuclear, chemical, and physical properties of these isotopes. A substantial amount of progress has been achieved in recent years in refining old and developing new techniques of measurement of the properties quoted, and isotope processing. This has resulted in a broad technological base from which both the material and information about the material is available. Un- fortunately, it has also resulted in a multiplicity of sources so that information and data are either untimely or present properties without adequately identifying the measurement techniques or describing the quality of material used. The purpose of this document is to make available, in a single reference, an annotated bibliography and sets of properties for nine of the more attractive isotopes available for use in power production. Part I contains all the unclassified information that was available in the literature surveyed. Part II is the classified counterpart to Part I. -

Measurement of Cerium in Human Breast Milk and Blood
Measurement of cerium in human breast milk and blood Vera Höllriegl, Montserrat Gonzáles-Estecha*, Elena M. Trasobares*, Augusto Giussani, Uwe Oeh, Miguel Angel Herraiz**, Bernhard Michalkea Institute of Radiation Protection and aInstitute of Ecological Chemistry, Helmholtz Zentrum München, German Research Center for Environmental Health, Ingolstädter Landstrasse 1, 85764 Neuherberg, Germany *Trace Element Unit, Department of Laboratory Medicine and **Department of Obstetrics and Gynaecology, Hospital Clinico Universitario San Carlos, 28040 Madrid, Spain Abstract The aim of this study was to evaluate the relationship between cerium content in human breast milk and blood plasma or serum and the influence due to the environmental exposure. Blood samples and breast milk at various stages of lactation, from 5 days to 51 weeks post partum, were donated by 42 healthy breast-feeding mothers from Munich, Germany and by 26 lactating Spanish mothers from Madrid at 4 weeks post partum. Inductively coupled plasma mass spectrometry was applied for the determination of cerium in the biological samples. Cerium concentration in the digested milk samples from Munich showed low values and the arithmetic mean values ranged between the quantification limit of 5 ng/L up to 65 ng/L. The median value amounted to 13 ng/L. The cerium concentrations in the Spanish breast milk samples amounted to similar low values. The data were about a factor of eight lower than values found in a former study of samples from an eastern German province. All cerium concentrations in the German plasma samples, except for two, were at the quantification limit of 10 ng/L. Interestingly, the serum samples of the Spanish mothers 1 showed cerium values ranging between 21.6 and 70.3 ng/L; these higher data could be explained by an enhanced intake of cerium by humans in Madrid. -

EPA Facts About Americium-241 July 2002
Argonne National Laboratory, EVS Human Health Fact Sheet, August 2005 Americium What Is It? Americium is a malleable, silvery white metal that tarnishes slowly in dry air at room temperature. Americium does not occur naturally but is produced artificially Symbol: Am by successive neutron capture reactions by plutonium isotopes. There are sixteen known isotopes of americium and all of them are radioactive. Atomic Number: 95 (Isotopes are different forms of an element that have the same number of (protons in nucleus) protons in the nucleus but a different number of neutrons.) Americium-241 was first produced in 1944 in a nuclear reactor at the University of Chicago. Atomic Weight: - Dr. Glenn Seaborg gave the new element its name in 1946 in honor of the (not naturally occurring) continent on which it was discovered. Of the sixteen radioactive isotopes, only three have half-lives long enough to warrant concern at Department of Energy (DOE) environmental management sites: americium-241, americium-242m, and americium-243. The half-lives of these three isotopes range from 150 to 7,400 Radioactive Properties of Key Americium Isotopes years, while those of the other and Associated Radionuclides Specific Radiation Energy (MeV) isotopes are less than a day. Half- Decay Isotope Activity Americium-241 is generally the Life Mode Alpha Beta Gamma most prevalent isotope at DOE (Ci/g) (α) (β) (γ) sites such as Hanford. It has a Am-241 430 yr 3.5 α 5.5 0.052 0.033 half-life of 430 years and decays Am-242m 150 yr 9.8 IT 0.025 0.044 0.0051 by emitting an alpha particle Am-242 16 hr 820,000 β, EC - 0.18 0.018 with attendant gamma radiation. -

Americium Cas #7440-35-9
AMERICIUM CAS #7440-35-9 Division of Toxicology ToxFAQsTM April 2004 This fact sheet answers the most frequently asked health questions (FAQs) about americium. For more information, call the ATSDR Information Center at 1-888-422-8737. This fact sheet is one in a series of summaries about hazardous substances and their health effects. It is important you understand this information because this substance may harm you. The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present. HIGHLIGHTS: Very low levels of americium occur in air, water, soil, and food, as well as in smoke detectors. Exposure to radioactive americium may result in increased cancer risk. Americium has been found in at least 8 of the 1,636 National Priorities List (NPL) sites identified by the Environmental Protection Agency (EPA). What is americium? ‘ Americium strongly sticks to soil particles and does not travel very far into the ground. Americium is a man-made radioactive chemical. Americium ‘ Plants may take up small amounts of americium from the has no naturally occurring or stable isotopes. Two soil. important isotopes of americium are americium 241 (241Am) ‘ Fish may take up americium, but little builds up in the (read as americium two-forty-one) and 243Am. Both isotopes fleshy tissue. In shellfish, americium is attached to the shell have the same chemical behavior in the environment and the and not to the parts you normally eat. same chemical effects on your body. How might I be exposed to americium? 241Americium is used in ionization smoke detectors. -
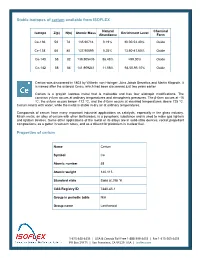
Stable Isotopes of Cerium Available from ISOFLEX Properties of Cerium
Stable isotopes of cerium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Ce-136 58 78 135.90714 0.19% 30.00-53.40% Oxide Ce-138 58 80 137.90599 0.25% 13.60-41.60% Oxide Ce-140 58 82 139.905435 88.48% >99.00% Oxide Ce-142 58 84 141.909241 11.08% 93.50-95.10% Oxide Cerium was discovered in 1803 by Wilhelm von Hisinger, Jöns Jakob Berzelius and Martin Klaproth. It is named after the asteroid Ceres, which had been discovered just two years earlier. Cerium is a grayish lustrous metal that is malleable and has four allotropic modifications. The common γ-form occurs at ordinary temperatures and atmospheric pressures. The β-form occurs at -16 ºC, the α-form occurs below -172 ºC, and the δ-form occurs at elevated temperatures above 725 ºC. Cerium reacts with water, while the metal is stable in dry air at ordinary temperatures. Compounds of cerium have many important industrial applications as catalysts, especially in the glass industry. Misch metal, an alloy of cerium with other lanthanides, is a pyrophoric substance and is used to make gas lighters and ignition devices. Some other applications of the metal or its alloys are in solid-state devices, rocket propellant compositions, as a getter in vacuum tubes, and as a diluent for plutonium in nuclear fuel. Properties of cerium Name Cerium Symbol Ce Atomic number 58 Atomic weight 140.115 Standard state Solid at 298 °K CAS Registry ID 7440-45-1 Group in periodic table N/A Group name Lanthanoid 1‐415‐440‐4433 │ USA & Canada Toll Free 1‐888‐399‐4433 -

An Investigation of the Isotopes of Americium and Curium
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title AN INVESTIGATION OF THE ISOTOPES OF AMERICIUM AND CURIUM Permalink https://escholarship.org/uc/item/9z7727pf Author Higgins, Gary Hoyt. Publication Date 2011-02-01 eScholarship.org Powered by the California Digital Library University of California UCRL 1796 Unclassified Chemistry Distribution UNIVERSITY OF CALIFORNIA Radiation Laboratory Contract No o W-7405=eng=48 AN INVESTIGATION OF THE ISOTOPES OF AMERICIUM AND CURIUM Gary Hoyt Higgins (Thesis) June» 1952 Berkeley 9 California. TABLE OF CONTENTS Page LIST OF ILLUSTRATIONS 3 ABSTRACT 4 Part I. INTRODUCTION . 5 II. EXPERIMENTAL METHODS 8 A. Bombardment Techniques 8 B. Chemical Procedures 8 C. Counting Techniques and Equipment . 10 III. RESULTS AND CONCLUSIONS 12 A. Am240 12 B. Am239 13 C. Am238 13 D. Am237 16 Eo Cm241 16 F. Cm240 19 G. Cm239 24 H. Cm238 27 I. General 29 ACKNOWLEDGNENTS 33 APPENDIX 34 REFERENCES 38 -2- LIST OF ILLUSTRATIONS Figure Page I. Pulse analysis of alpha particles of americium produced by bombarding Pu239 with 19 Mev deuterons . • 0 14 2. Decay of americium produced by bombardment of pu239 with 19 Mev ,deuterons 15 3. Decay of americium produced by bombardment of pu239 wi th 30--50 Mev deuterons 17 4. Alpha pulse analysis of particles from americium produced by bombarding Pu239 with 30-50 Mev deuterons. 18 5. Decay of curium produced by bombarding pu239 with 27 Mev helium ions 20 6. Alpha pulse analysis of curium produced by bombarding pu239 with 27 Mev helium ions . • . .. 2l 7. Dec~y of curium produced by bombarding Pu239 with 38 Mev helium ions 22 8. -

Separation of Cerium from Lanthanum
Banha University Faculty of Science Chemistry Department Radiochemical Study on the Medically and Technologically Radionuclides of Some Lanthanides Presented by Hany Abd ElEl----HamidHamid Abd ElEl----AzizAziz Aglan Egyption Atomic Energy Authority Nuclear Research Center Cyclotron Project For The degree of Master of Science in Chemistry (Physical chemistry) Supervised by Prof. Dr. Mahmoud Ahmed Mousa Prof. Dr. Zeinab Abdou Saleh Professor of Physical Chemistry Professor of Nuclear Physics Faculty of Science Nuclear Research Center Benha University Atomic Energy Authority Dr. Hassan Ali Hanafi Lecturer of Physical and Applied Chemistry Cyclotron Project Nuclear Research Center Atomic Energy Authority 2010 Approval Sheet Title : Radiochemical Study on the Medically and Technologically Radionuclides of Some Lanthanides Name: Hany Abd ElEl----HamidHamid Abd ElEl----AzizAziz Aglan Supervisors: Name Position Signature Prof. Dr. Mahmoud Ahmed Professor of Physical Mousa Chemistry - Benha University Prof. Dr. Zeinab Abdou Professor of Nuclear Physics Saleh Atomic Energy Authority Dr. Hassan Ali Hanafi Lecturer of Physical and Applied Chemistry - Atomic Energy Authority Head of Chemistry Vice – Dean Dean of Faculty Department for Graduate Studies and Research Prof. Dr. S. G. Donia Prof. Dr. M. A. El-Fakharany REFEREES DECISION Title: Radiochemical Study on the Medically and Technologically Radionuclides of Some Lanthanides Name: Hany Abd El El----HamidHamid Abd ElEl----AzizAziz Aglan Referees: Name Position Signature Date of Discussion: Degree -

Nuclear Terrorism
Nuclear terrorism Jan Willem Storm van Leeuwen Independent consultant member of the Nuclear Consulting Group July 2019 [email protected] Note In this document the references are coded by Q-numbers (e.g. Q6). Each reference has a unique number in this coding system, which is consistently used throughout all publications by the author. In the list at the back of the document the references are sorted by Q-number. The resulting sequence is not necessarily the same order in which the references appear in the text. m23terrorism20190719 1 Contents 1 Nuclear ex[losives Uranium Enrichment of uranium Uranium-233 Plutonium Neptunium-237 Americium 2 Nuclear terrorism Threats MOX fuel Safeguards of plutonium Safeguards of HEU and uranium-233 Safeguards of neptunium and americium Dirty bomb 3 Illicit trafficking and theft Failing nuclear supervision Uncontrollable transports of nuclear materials Illegal dumping at sea 4 Nuclear security and reprocessing of spent fuel Separation of fissile materials Roots of reprocessing Security issues of the breeder and P&T cycles Benefits of reprocessing Keep spent fuel elements intact 5 Vulnerability of nuclear installations Mass casualty attacks Armed conflicts Natural disasters m23terrorism20190719 2 1 Nuclear explosives Uranium An atomic bomb can be made from materials containing sufficient fissile nuclides to sustain a divergent fission chain reaction. Uranium as found in nature contains 0.7% uranium-235, the only fissile nuclide occurring in nature. The remaining 99.3% consists of U-238 and traces of U-234, both nuclides are not fissile. Natural uranium is not suitable for bombs, it has to be enriched in U-235 to make a nuclear explosion possible. -

Production, Study and Us© of in Pure and Applied Nuclear Research
Production, study and us© of In pure and applied nuclear research by Tor Bjernstad University of Bergen 1986 THESIS for the degree Doctor Philosophiae (dr.philos.) at the University of Oslo. Obligatoric lectures: April 25, 1986. Defence of the thesis: April 26, 1986. to tlie thesis PRODUCTION, STUDI AND USE Of SHORT-LIVED NUCLIUES IH PURE AND APPLIED NUCLEAR RESEARCH by lor BJornstad LOCA!ION IS WRITTEN SHOULD BE 238,, • 235,, REVIEW PAPER, (>.)), line II "...Induced fission of U..." "...Induced fission of U..." REVIEW PAPER, p.14, Une 4 "Chemical metods..." "Chemical methods.,." SEVIEW PAPER, p.JO, line 17 "lived mud ides ("or..." "livpd nuclides for..." REVIEW PAPER, p.29, line 11 '...for sampling of this,.," "...for sampling of thin..." REVIEW PAPEfl, p.30, line 26 \..T1/2(M) * Tl/JID)..." \..Tj{H) * Tj(D)..." REVIEW PAPER, p.31, line 11 "...suncrocyclotron..," "... synchrocyclotron.*." REVIEW PAPER, p.31. line B **... Trie release tfie »uclear..." "...The release of the nuclear. from the bottom REVIEW PAPER, p.31. line A "...low vapor pressure..." ..low vapour pressure., from the bottom REVIEW PAPER, p.35, line •'...76Or(tj-1.355)..." \..76V(tj-1.3Ss)..." REVIEV PAPER, p.47, 1 lues 6, Ptiranthese after the reference on These parflntfieses should be on the 11,1? and 14 ttje level of Hie text line level of the reference number. In addition, there should be Inserted connid between references in line 15 and 12. REVIEW PAPER, p.48. line 1 "The separted molecular. "The separated molecular,.," PfVIEW PAPER, p.48, line 7 "...irridfum..." "...iridium..." from the bottom REVIEW PACER, p.50.