Comparing Antithrombotic Strategies After Bioprosthetic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Low Molecular Weight Heparin Versus Acenocoumarol in the Secondary Prophylaxis of Deep Vein Thrombosis
Thromb Haemost 1999; 81: 26–31 © 1999 Schattauer Verlag, Stuttgart Low Molecular Weight Heparin versus Acenocoumarol in the Secondary Prophylaxis of Deep Vein Thrombosis S. Lopaciuk, H. Bielska-Falda1, W. Noszczyk1, M. Bielawiec 2, W. Witkiewicz 3, S. Filipecki 4, J. Michalak 5, L. Ciesielski 6, Z. Mackiewicz 7, E. Czestochowska 8, K. Zawilska 9, A. Cencora 10, among others* From the Institute of Hematology and Blood Transfusion, Warsaw, 1st 1Department of Surgery, Medical School, Warsaw, 2Department of Hematology, Medical School, Bialystok, 3Department of Vascular Surgery, District General Hospital, Wroclaw, 4Department of Internal Medicine, Institute of Tuberculosis and Lung Diseases, Warsaw, 5Department of Vascular Surgery, Medical School, Lublin, 1st 6Department of Surgery, Medical School, Lodz, 7Department of Vascular Surgery, Medical School, Bydgoszcz, 3rd 8Department of Internal Medicine, Medical School, Gdansk, 9Department of Hematology, Medical School, Poznan, and 10Department of Vascular Surgery, District General Hospital, Krakow, Poland Summary oral anticoagulant for at least 3 months (secondary prophylaxis). Al- though long-term anticoagulation with coumarin drugs, the most popu- The aim of this study was to determine the efficacy and safety of lar of which are warfarin and acenocoumarol, has been repeatedly dem- subcutaneous weight-adjusted dose low molecular weight heparin onstrated effective in the prevention of recurrent venous thromboembo- (LMWH) compared with oral anticoagulant (OA) in the prevention of lism, it suffers from a number of limitations. This treatment requires recurrent venous thromboembolism. In a prospective multicenter trial, strict laboratory control and consequent adjustments of the drug dosage 202 patients with symptomatic proximal deep vein thrombosis (DVT) and carries a substantial risk of bleeding complications (1). -

(12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao Et Al
USOO9498481 B2 (12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao et al. (45) Date of Patent: *Nov. 22, 2016 (54) CYCLOPROPYL MODULATORS OF P2Y12 WO WO95/26325 10, 1995 RECEPTOR WO WO99/O5142 2, 1999 WO WOOO/34283 6, 2000 WO WO O1/92262 12/2001 (71) Applicant: Apharaceuticals. Inc., La WO WO O1/922.63 12/2001 olla, CA (US) WO WO 2011/O17108 2, 2011 (72) Inventors: Tadimeti Rao, San Diego, CA (US); Chengzhi Zhang, San Diego, CA (US) OTHER PUBLICATIONS Drugs of the Future 32(10), 845-853 (2007).* (73) Assignee: Auspex Pharmaceuticals, Inc., LaJolla, Tantry et al. in Expert Opin. Invest. Drugs (2007) 16(2):225-229.* CA (US) Wallentin et al. in the New England Journal of Medicine, 361 (11), 1045-1057 (2009).* (*) Notice: Subject to any disclaimer, the term of this Husted et al. in The European Heart Journal 27, 1038-1047 (2006).* patent is extended or adjusted under 35 Auspex in www.businesswire.com/news/home/20081023005201/ U.S.C. 154(b) by Od en/Auspex-Pharmaceuticals-Announces-Positive-Results-Clinical M YW- (b) by ayS. Study (published: Oct. 23, 2008).* This patent is Subject to a terminal dis- Concert In www.concertpharma. com/news/ claimer ConcertPresentsPreclinicalResultsNAMS.htm (published: Sep. 25. 2008).* Concert2 in Expert Rev. Anti Infect. Ther. 6(6), 782 (2008).* (21) Appl. No.: 14/977,056 Springthorpe et al. in Bioorganic & Medicinal Chemistry Letters 17. 6013-6018 (2007).* (22) Filed: Dec. 21, 2015 Leis et al. in Current Organic Chemistry 2, 131-144 (1998).* Angiolillo et al., Pharmacology of emerging novel platelet inhibi (65) Prior Publication Data tors, American Heart Journal, 2008, 156(2) Supp. -

Occurrence, Elimination, and Risk of Anticoagulant Rodenticides in Wastewater and Sludge
Occurrence, elimination, and risk of anticoagulant rodenticides in wastewater and sludge Silvia Lacorte, Cristian Gómez- Canela Department of Environmental Chemistry, IDAEA-CSIC, Jordi Girona 18-26, 08034 Barcelona Rats and super-rats Neverending story 1967 Coumachlor 1 tn rodenticides /city per campaign “It will be the LAST ONE” Rodenticides Biocides: use regulated according to EU. Used mainly as bait formulations. First generation: multiple feedings, less persistent in tissues, commensal and outdoor use. Second generation: single feeding (more toxic), more persistent in tissue, commensal use only. Toxic: vitamin K antagonists that cause mortality by blocking an animal’s ability to produce several key blood clotting factors. High oral, dermal and inhalation toxicity. Origin and fate of rodenticides Study site: Catalonia (7.5 M inhabitants) 1693 km of sewage corridor 13 fluvial tanks (70.000 m3) 130,000,000 € / 8 YEARS 32,000 km2 378,742 kg/y AI 2,077,000 € Objectives 1. To develop an analytical method to determine most widely used rodenticides in wastewater and sludge. 2. To monitor the presence of rodenticides within 9 WWTP receiving urban and agricultural waters. 3. To evaluate the risk of rodenticides using Daphnia magna as aquatic toxicological model. 4. To study the accumulation of rodenticides in sludge. Compounds studied Coumachlor* Pindone C19H15ClO4 C14H14O3 Dicoumarol Warfarin C19H12O6 C19H16O4 Coumatetralyl Ferulenol FGARs C19H16O3 C24H30O3 Acenocoumarol Chlorophacinone • Solubility C19H15NO6 C23H15ClO3 0.001-128 mg/L • pKa 3.4-6.6 Flocoumafen Bromadiolone C H F O C H BrO 33 25 3 40 30 23 4 • Log P 1.92-8.5 Brodifacoum Fluindione C H BrO 31 23 3 C15H9FO2 SGARs Difenacoum Fenindione C31H24O3 C15H10O2 1. -
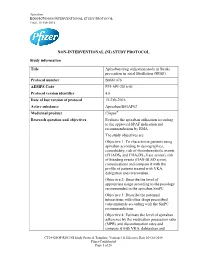
STUDY PROTOCOL Study Information Title Apixaban Drug
Apixaban B0661076NON-INTERVENTIONAL STUDY PROTOCOL Final, 15-Feb-2016 NON-INTERVENTIONAL (NI) STUDY PROTOCOL Study information Title Apixaban drug utilization study in Stroke prevention in atrial fibrillation (SPAF) Protocol number B0661076 AEMPS Code PFI-API-2016-01 Protocol version identifier 4.0 Date of last version of protocol 15-Feb-2016 Active substance Apixaban B01AF02 Medicinal product Eliquis® Research question and objectives Evaluate the apixaban utilization according to the approved SPAF indication and recommendations by EMA. The study objectives are: Objective 1: To characterise patients using apixaban according to demographics, comorbidity, risk of thromboembolic events (CHADS2 and CHA2DS2-Vasc scores), risk of bleeding events (HAS-BLED score), comedications and compare it with the profile of patients treated with VKA, dabigatran and rivaroxaban. Objective 2: Describe the level of appropriate usage according to the posology recommended in the apixaban SmPC. Objective 3: Describe the potential interactions with other drugs prescribed concomitantly according with the SmPC recommendations. Objective 4: Estimate the level of apixaban adherence by the medication possession ratio (MPR) and discontinuation rates and compare it with VKA, dabigatran and CT24-GSOP-RF03 NI Study Protocol Template; Version 3.0, Effective Date 10-Oct-2014 Pfizer Confidential Page 1 of 28 Apixaban B0661076NON-INTERVENTIONAL STUDY PROTOCOL Final, 15-Feb-2016 rivaroxaban cohort. Objective 5: To analyze INR (International Normalized Ratio) values during the last 12 months and to obtain TTR (Time in Therapeutic Range) values in patients previously treated with VKA and, during the whole study period for those in the cohort treated with VKA Author Ángeles Quijada Manuitt, IDIAP Jordi Gol Rosa Morros Pedrós, IDIAP Jordi Gol Jordi Cortés, IDIAP Jordi Gol José Chaves Puertas, Pfizer SLU Sponsor Pfizer S.L.U Avda. -

Review of Anticoagulant Drugs in Paediatric Thromboembolic Disease
18th Expert Committee on the Selection and Use of Essential Medicines (21 to 25 March 2011) Section 12: Cardiovascular medicines 12.5 Antithrombotic medicines Review of Anticoagulant Drugs in Paediatric Thromboembolic Disease September 2010 Prepared by: Fiona Newall Anticoagulation Nurse Manager/ Senior Research Fellow Royal Children’s Hospital/ The University of Melbourne Melbourne, Australia 1 Contents Intent of Review Review of indications for antithrombotic therapy in children Venous thromboembolism Arterial thrombeombolism Anticoagulant Drugs Unfractionated heparin Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations Vitamin K antagonists Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations Low Molecular Weight Heparins Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations ??Novel anticoagulants Summary 2 1. Intent of Review To review the indications for anticoagulant therapies in children. To review the epidemiology of thromboembolic events in children. To review the literature and collate the evidence regarding dosing, administration and monitoring of anticoagulant therapies in childhood. To review the safety of anticoagulant therapies and supervision required To give recommendations for the inclusion of anticoagulants on the WHO Essential Medicines List 2. Review of indications for antithrombotic therapy in children Anticoagulant therapies are given for the prevention or treatment of venous and/or arterial thrombosis. The process of ‘development haemostasis’, whereby the proteins involved in coagulation change quantitatively and qualitatively with age across childhood, essentially protects children against thrombosis(1-5). For this reason, insults such as immobilization are unlikely to trigger the development of thrombotic diseases in children. -

Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-Target Organisms Katherine Horak U.S
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Plant Publications Health Inspection Service 2018 Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms Katherine Horak U.S. Department of Agriculture, [email protected] Penny M. Fisher Landcare Research Brian M. Hopkins Landcare Research Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Part of the Life Sciences Commons Horak, Katherine; Fisher, Penny M.; and Hopkins, Brian M., "Pharmacokinetics of Anticoagulant Rodenticides in Target and Non- target Organisms" (2018). USDA National Wildlife Research Center - Staff Publications. 2091. https://digitalcommons.unl.edu/icwdm_usdanwrc/2091 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff ubP lications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Chapter 4 Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms Katherine E. Horak, Penny M. Fisher, and Brian Hopkins 1 Introduction The concentration of a compound at the site of action is a determinant of its toxicity. This principle is affected by a variety of factors including the chemical properties of the compound (pKa, lipophilicity, molecular size), receptor binding affinity, route of exposure, and physiological properties of the organism. Many compounds have to undergo chemical changes, biotransformation, into more toxic or less toxic forms. Because of all of these variables, predicting toxic effects and performing risk assess- ments of compounds based solely on dose are less accurate than those that include data on absorption, distribution, metabolism (biotransformation), and excretion of the compound. -

New Zealand Data Sheet 1
New Zealand Data Sheet 1. PRODUCT NAME NAXEN® 250 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each NAXEN 250 mg tablet contains 250 mg of Naproxen For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM NAXEN 250 mg tablets are yellow, biconvex, round tablet of 11 mm diameter with one face engraved NX250 and having a bisecting score. The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications NAXEN is indicated in adults for the relief of symptoms associated with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, tendonitis and bursitis, acute gout and primary dysmenorrhoea. NAXEN is indicated in children for juvenile arthritis. 4.2. Dose and method of administration After assessing the risk/benefit ratio in each individual patient, the lowest effective dose for the shortest possible duration should be used (see Section 4.4). During long-term administration the dose of naproxen may be adjusted up or down depending on the clinical response of the patient. A lower daily dose may suffice for long-term administration. In patients who tolerate lower doses well, the dose may be increased to 1000 mg per day when a higher level of anti-inflammatory/analgesic 1 | P a g e activity is required. When treating patients with naproxen 1000 mg/day, the physician should observe sufficient increased clinical benefit to offset the potential increased risk. Dose Adults For rheumatoid arthritis, osteoarthritis and ankylosing spondylitis Initial therapy: The usual dose is 500-1000 mg per day taken in two doses at 12 hour intervals. -

Heavy Rainfall Provokes Anticoagulant Rodenticides' Release from Baited
Journal Pre-proof Heavy rainfall provokes anticoagulant rodenticides’ release from baited sewer systems and outdoor surfaces into receiving streams Julia Regnery1,*, Robert S. Schulz1, Pia Parrhysius1, Julia Bachtin1, Marvin Brinke1, Sabine Schäfer1, Georg Reifferscheid1, Anton Friesen2 1 Department of Biochemistry, Ecotoxicology, Federal Institute of Hydrology, 56068 Koblenz, Germany 2 Section IV 1.2 Biocides, German Environment Agency, 06813 Dessau-Rosslau, Germany *Corresponding author. Email: [email protected] (J. Regnery); phone: +49 261 1306 5987 Journal Pre-proof A manuscript prepared for possible publication in: Science of the Total Environment May 2020 1 Journal Pre-proof Abstract Prevalent findings of anticoagulant rodenticide (AR) residues in liver tissue of freshwater fish recently emphasized the existence of aquatic exposure pathways. Thus, a comprehensive wastewater treatment plant and surface water monitoring campaign was conducted at two urban catchments in Germany in 2018 and 2019 to investigate potential emission sources of ARs into the aquatic environment. Over several months, the occurrence and fate of all eight ARs authorized in the European Union as well as two pharmaceutical anticoagulants was monitored in a variety of aqueous, solid, and biological environmental matrices during and after widespread sewer baiting with AR-containing bait. As a result, sewer baiting in combined sewer systems, besides outdoor rodent control at the surface, was identified as a substantial contributor of these biocidal active ingredients in the aquatic environment. In conjunction with heavy or prolonged precipitation during bait application in combined sewer systems, a direct link between sewer baiting and AR residues in wastewater treatment plant influent, effluent, and the liver of freshwater fish was established. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Perioperative Management of Patients Treated with Antithrombotics in Oral Surgery
SFCO/Perioperative management of patients treated with antithrombotic agents in oral surgery/Rationale/July 2015 SOCIÉTÉ FRANÇAISE DE CHIRURGIE ORALE [FRENCH SOCIETY OF ORAL SURGERY] IN COLLABORATION WITH THE SOCIÉTÉ FRANÇAISE DE CARDIOLOGIE [FRENCH SOCIETY OF CARDIOLOGY] AND THE PERIOPERATIVE HEMOSTASIS INTEREST GROUP Space Perioperative management of patients treated with antithrombotics in oral surgery. RATIONALE July 2015 P a g e 1 | 107 SFCO/Perioperative management of patients treated with antithrombotic agents in oral surgery/Rationale/July 2015 Abbreviations ACS Acute coronary syndrome(s) ADP Adenosine diphosphate Afib Atrial Fibrillation AHT Arterial hypertension Anaes Agence nationale d’accréditation et d’évaluation en santé [National Agency for Accreditation and Health Care Evaluation] APA Antiplatelet agent(s) aPTT Activated partial thromboplastin time ASA Aspirin BDMP Blood derived medicinal products BMI Body mass index BT Bleeding Time cAMP Cyclic adenosine monophosphate COX-1 Cyclooxygenase 1 CVA Cerebral vascular accident DIC Disseminated intravascular coagulation DOA Direct oral anticoagulant(s) DVT Deep vein thrombosis GEHT Study Group on Hemostasis and Thrombosis (groupe d’étude sur l’hémostase et la thrombose) GIHP Hemostasis and Thrombosis Interest Group (groupe d’intérêt sur l’hémostase et la thrombose) HAS Haute autorité de santé [French Authority for Health] HIT Heparin-induced thrombocytopenia IANB Inferior alveolar nerve block INR International normalized ratio IV Intravenous LMWH Low-molecular-weight heparin(s) -

(12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub
US 20090005722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub. Date: Jan. 1, 2009 (54) SKIN-CONTACTING-ADHESIVE FREE Publication Classification DRESSING (51) Int. Cl. Inventor: Barbara Jennings-Spring, Jupiter, A61N L/30 (2006.01) (76) A6F I3/00 (2006.01) FL (US) A6IL I5/00 (2006.01) Correspondence Address: AOIG 7/06 (2006.01) Irving M. Fishman AOIG 7/04 (2006.01) c/o Cohen, Tauber, Spievack and Wagner (52) U.S. Cl. .................. 604/20: 602/43: 602/48; 4771.5; Suite 2400, 420 Lexington Avenue 47/13 New York, NY 10170 (US) (57) ABSTRACT (21) Appl. No.: 12/231,104 A dressing having a flexible sleeve shaped to accommodate a Substantially cylindrical body portion, the sleeve having a (22) Filed: Aug. 29, 2008 lining which is substantially non-adherent to the body part being bandaged and having a peripheral securement means Related U.S. Application Data which attaches two peripheral portions to each other without (63) Continuation-in-part of application No. 1 1/434,689, those portions being circumferentially adhered to the sleeve filed on May 16, 2006. portion. Patent Application Publication Jan. 1, 2009 Sheet 1 of 9 US 2009/0005722 A1 Patent Application Publication Jan. 1, 2009 Sheet 2 of 9 US 2009/0005722 A1 10 8 F.G. 5 Patent Application Publication Jan. 1, 2009 Sheet 3 of 9 US 2009/0005722 A1 13 FIG.6 2 - Y TIII Till "T fift 11 10 FIG.7 8 13 6 - 12 - Timir" "in "in "MINIII. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01