Geoarch Report 2010/20
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biodiversity Opportunity Areas: the Basis for Realising Surrey's Local
Biodiversity Opportunity Areas: The basis for realising Surrey’s ecological network Surrey Nature Partnership September 2019 (revised) Investing in our County’s future Contents: 1. Background 1.1 Why Biodiversity Opportunity Areas? 1.2 What exactly is a Biodiversity Opportunity Area? 1.3 Biodiversity Opportunity Areas in the planning system 2. The BOA Policy Statements 3. Delivering Biodiversity 2020 - where & how will it happen? 3.1 Some case-studies 3.1.1 Floodplain grazing-marsh in the River Wey catchment 3.1.2 Calcareous grassland restoration at Priest Hill, Epsom 3.1.3 Surrey’s heathlands 3.1.4 Priority habitat creation in the Holmesdale Valley 3.1.5 Wetland creation at Molesey Reservoirs 3.2 Summary of possible delivery mechanisms 4. References Figure 1: Surrey Biodiversity Opportunity Areas Appendix 1: Biodiversity Opportunity Area Policy Statement format Appendix 2: Potential Priority habitat restoration and creation projects across Surrey (working list) Appendices 3-9: Policy Statements (separate documents) 3. Thames Valley Biodiversity Opportunity Areas (TV01-05) 4. Thames Basin Heaths Biodiversity Opportunity Areas (TBH01-07) 5. Thames Basin Lowlands Biodiversity Opportunity Areas (TBL01-04) 6. North Downs Biodiversity Opportunity Areas (ND01-08) 7. Wealden Greensands Biodiversity Opportunity Areas (WG01-13) 8. Low Weald Biodiversity Opportunity Areas (LW01-07) 9. River Biodiversity Opportunity Areas (R01-06) Appendix 10: BOA Objectives & Targets Summary (separate document) Written by: Mike Waite Chair, Biodiversity Working Group Biodiversity Opportunity Areas: The basis for realising Surrey’s ecological network, Sept 2019 (revised) 2 1. Background 1.1 Why Biodiversity Opportunity Areas? The concept of Biodiversity Opportunity Areas (BOAs) has been in development in Surrey since 2009. -

The Beeches, 9 South Farm Lane, Bagshot, Surrey the Beeches Garages
The Beeches, 9 South Farm Lane, Bagshot, Surrey The Beeches garages. The kitchen offers a range of contemporary wall and floor units with a large 9 South Farm Lane, central island, wooden worktops, a Belfast sink, Bagshot, Surrey modern integrated appliances and space for a dining and breakfast table. GU19 5NT The first floor accommodation provides a spacious dual aspect main bedroom with built- A handsome period property with in storage and a large contemporary en suite self-contained studio annexe, set in just bathroom with freestanding slipper bath and under half an acre of beautiful grounds. separate walk-in shower, two further bedrooms, one with en suite bathroom. The property’s M3 (Jct. 3) 0.3 mile, Bagshot station 0.9 mile two remaining bedrooms can be found on the (London Waterloo 1 hour 12 minutes), Lightwater second floor, together with a family shower 0.8 mile, Windlesham 1.4 miles, Ascot 4.4 miles, room. London Heathrow Airport 13.7 miles, central London 30.8 miles Outside The property is approached through double Porch | Reception hall | Reception room | Sitting electric wooden gates with a side pedestrian room | Study | Conservatory | Store room gate over a gravelled driveway providing Kitchen/breakfast room | Utility room | Inner extensive private parking and giving access to hallway | 2 Cloakrooms | Main bedroom with en the inter-connecting double and single garages suite bathroom | Guest bedroom with en suite with first floor studio annexe over. The annexe 3 Further bedrooms | Family shower room offers a large living room with kitchenette and Double garage | Single garage | Garden | Self- a spacious en suite bathroom with freestanding contained studio annexe with kitchenette and en bath and separate walk-in shower. -

Bourne Holdings, Broadway Road, Windlesham
2018/0709 Reg Date 07/08/2018 Lightwater LOCATION: BOURNE HOLDINGS, BROADWAY ROAD, WINDLESHAM, LIGHTWATER, GU18 5SH PROPOSAL: Removal of condition 7 of 94/0998 requiring agricultural occupancy of bungalow at Bourne Holdings and discharge of section 52 agreement under 87/1324 restricting use of the site to agriculture and the parking of two HGV vehicles. TYPE: Relaxation/Modification APPLICANT: Ms Gostage OFFICER: Ross Cahalane The application would normally be determined under the Council’s Scheme of Delegation, however, it has been called in for determination by the Planning Applications Committee at the request of Cllr Gandhum, as the property is subject to an agricultural tie. RECOMMENDATION: GRANT 1.0 SUMMARY 1.1 This application seeks permission for the removal of Condition 7 of 94/0998 requiring agricultural occupancy of the bungalow at Bourne Holdings. This condition requires occupants of the dwelling to be employed locally in agriculture or in forestry. The application also seeks discharge of the Section 52 legal agreement under 87/1324, which restricts the use of the site as a whole to agriculture and the parking of two HGV vehicles. No operational development is proposed. 1.2 The report concludes that as no interest from persons compliant with the occupancy terms required by the planning condition has been identified through the marketing of the property for sale, Bourne Holdings no longer requires an imposed occupancy condition as part of 94/0998. The dwelling no longer forms part of an agricultural holding since 2007, nor is it considered required for occupation by a person employed in agriculture in the local area. -

AMR200910.Pdf
This page has been left blank deliberately FOREWORD The Surrey Heath Annual Monitoring Report (AMR) is published each year. This Report monitors the period 1st April 2009 to 31st March 2010. It sets out the progress achieved in implementing the Local Development Framework (LDF) and performance against the policies of the Surrey Heath Local Plan 2000 and the Core Output Indicators relating to development plans. This is the sixth statutory Surrey Heath AMR and will be submitted to the Secretary of State by 31st December 2010. This is in accordance with Section 35 of the Planning and Compulsory Purchase Act 2004, which requires every Local Authority to submit an AMR to the Secretary of State. Contact Details Planning Policy and Conservation Team Surrey Heath Borough Council Surrey Heath House Knoll Road Camberley Surrey GU15 3HD Telephone: 01276 707100 E-mail: [email protected] i This page has been left blank deliberately Surrey Heath Borough Council – Annual Monitoring Report 2009/10 CONTENTS Page EXECUTIVE SUMMARY 2 CHAPTER 1 : INTRODUCTION 5 CHAPTER 2: SPATIAL PORTRAIT OF SURREY HEATH (PART A) 7 CHAPTER 3: PROGRESS OF THE LDF (PART B) 12 CHAPTER 4: MONITORING POLICIES IN THE SURREY HEATH 19 LOCAL PLAN 2000 (PART C) CHAPTER 5: GENERAL POLICIES 22 CHAPTER 6: URBAN ENVIRONMENT 33 CHAPTER 7: HERITAGE 35 CHAPTER 8: RURAL ENVIRONMENT AND BIODIVERSITY 38 CHAPTER 9: RECREATION 49 CHAPTER 10: HOUSING 51 CHAPTER 11: EMPLOYMENT 70 CHAPTER 12: SHOPPING 75 CHAPTER 13: MOVEMENT 79 CHAPTER 14: COMMUNITY SERVICES 83 CHAPTER 15: CAMBERLEY TOWN CENTRE 86 APPENDIX 1: CONTEXTUAL INFORMATION 90 APPENDIX 2: HOUSING COMPLETIONS 1st April 2009 – 31st 109 March 2010 APPENDIX 3: EMPLOYMENT COMPLETIONS OF 50+ SQM 111 2009/10 Surrey Heath Borough Council – Annual Monitoring Report 2009/10 EXECUTIVE SUMMARY a) This is the sixth statutory Surrey Heath Annual Monitoring Report (AMR) and will be submitted to the Secretary of State by 31st December 2010. -
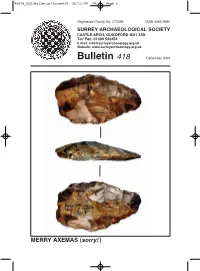
Bulletin/Issue418 26/11/09 09:46 Page 1
86476_SAS/Bulletin/Issue418 26/11/09 09:46 Page 1 Registered Charity No: 272098 ISSN 0585-9980 SURREY ARCHAEOLOGICAL SOCIETY CASTLE ARCH, GUILDFORD GU1 3SX Tel/ Fax: 01483 532454 E-mail: [email protected] Website: www.surreyarchaeology.org.uk Bulletin 418 December 2009 MERRY AXEMAS (sorry! ) 86476_SAS/Bulletin/Issue418 26/11/09 09:46 Page 2 Frontispiece: Palaeolith from the Farnham gravel terraces. Photo by Alan Hall to whom I owe an apology for omitting the scale (it wouldn’t fit the page). (see p17) ASHTEAD ROMAN VILLA AND TILEWORKS David Bird The fourth main season of excavation on Ashtead Common was undertaken by the Society’s Roman Studies Group between 26th August and 14th September. The weather was very kind and a larger digging team could be accommodated than previously because work took place in well-separated areas (numbers are restricted by nature conservation requirements). As a result, it was possible to achieve all the objectives for the year and indeed exceed them. The enclosure wall for the villa was found to extend at least as far west as just to the north of the bath-house attached to the villa; the phasing of the villa is now much better understood; a rough tile-paved area was found that may prove to be part of a tileworks structure; and the remnants of a tile kiln were found. As before, trench supervisors were David Calow, Nikki Cowlard and Frank Pemberton; Alan Hall controlled site recording and Margaret Broomfield was finds supervisor. Many other team regulars play a crucial part. -

Surrey Walks Club
Surrey Walks Club Walking for over 50 Years Affiliated to the Ramblers Affiliated to HF Holidays PROGRAMME January - March 2018 Chairman David Martin 01483 232668 Treasurer Secretary Andrew Campbell Fiona Ross 01932 840764 Walks Organiser David Underwood Committee Member Committee Member 01784 456775 Jenny Underwood Anneliese Cawthorne Walks Programme Sub-Committee Membership David Underwood Secretary Andrew Campbell Mike Smith Peter Horwood Clare Leeming Pauline Lamb Peter Weatherhead 01932 853056 ANNUAL SUBSCRIPTION - £12 www.surreywalksclub.org.uk NOTES FOR WALKERS MEETING POINTS AND TIMES We normally lift share to the start of walks and meet at the times shown in the programme as follows: Thursday Walks - At Coronation Recreation Ground free car park, Molesey Road, Hersham All Other Walks - At Walton-on-Thames Station main car park, Station Avenue (not the station forecourt, but the car park next to the Audi Garage) Or Alternatively - At the start of the walk, by prior arrangement with the Walk’s Leader. PROGRAMME CHANGES Details of any change of leaders or walks will be available from the club’s website www.surreywalksclub.org.uk or from the Walks’ Organiser. TRAVEL TO STARTING POINT By car. Cars are optionally filled at the meeting place. Walkers without cars will be given seats. Passengers are expected to contribute towards the cost of fuel to the driver. WALKS DESCRIPTIONS M = morning, WDM = Whole Day Medium, WDL= Whole Day Long, HD = Half Day, E = Evening, LEIS = Leisurely (at a slower pace). WALKS GRADES SUGGESTED MILEAGE RATES PER PASSENGER G1 = Hilly, fairly strenuous 20 miles round trip £1.60 G2 = Gentle hills, moderate walking 30 miles round trip £2.40 G3 = Mainly flat, easy walking 40 miles round trip £3.20 + = Slightly more strenuous than 60 miles round trip £4.80 normal grade LUNCH STOPS It is always advisable to carry food and drink as many lunch stops are in the countryside. -

Core Strategy & Development Management Policies
Core Strategy & Development Management Policies Proposed Submission Document July 2010 Great Place • Great Community • Great Future Core Strategy and Development Management Policies Development Plan Document Proposed Submission Document 1 Surrey Heath Core Strategy and Development Management Pre-Submission Document 2010 Regional Spatial Strategy At the time of drafting this document the government had clearly signalled its intention to abolish Regional Spatial Strategies (RSS). The RSS for the south east is the “South East Plan”. Whilst the announcement of intention to abolish is a material consideration, the South East Plan remains at this time a part of the development plan for Surrey Heath. In taking the Core Strategy and Development Management Policies Development Plan Document forward to pre-submission the Council has had regard to the announcement by the Secretary of State and advice from the Planning Inspectorate. Accordingly it has considered which of its policies turn on or refer to South East Plan policy. In respect of policies for the economy, employment, retail, infrastructure, green infrastructure and biodiversity whilst these may rest on South East Plan policy the evidence base behind those policies together with the Councils own evidence base, is considered sufficient to support and justify on a local basis the policy approach being taken in this document in respect of these matters. In respect of housing land supply the Council has decided not to continue with the housing targets contained in the South East Plan. This reflects the Councils long standing view that whilst a high level of need for housing exists the constraints within the Borough make it difficult to accommodate this need without harm to the environment and in particular the Thames Basin Heaths Special Protection Area. -
Windlesham Neighbourhood Plan 2018-2028
Windlesham Neighbourhood Plan 2018-2028 Windlesham Parish Council The Council Offices, Lightwater Contents INTRODUCTION ................................................................................................................... 3 Foreword from the Parish Council .............................................................................. 3 A Neighbourhood Plan – Defined ............................................................................... 4 How Windlesham benefits from a Plan ...................................................................... 4 Restrictions to the Neighbourhood Plan process ....................................................... 5 How the Plan was prepared ........................................................................................ 6 THE DEVELOPMENT OF WINDLESHAM ............................................................................... 8 Growth and Nature ..................................................................................................... 8 THE PRESENT SITUATION .................................................................................................... 9 The Setting .................................................................................................................. 9 Population ................................................................................................................... 9 Employment and Travel ............................................................................................ 10 Transportation ......................................................................................................... -

Windlesham Neighbourhood Plan Consultation Statement
Windlesham Neighbourhood Plan Consultation Statement Windlesham Parish Council The Council Offices, Lightwater Contents Background .......................................................................................................................... 2 Summary .............................................................................................................................. 2 The Consultation Timeline ................................................................................................... 3 Annex 1 - Regulation 16…………………………………………………………………………………………………..5 Appendices ........................................................................................................................... 6 Appendix 1 ................................................................................................................... 7 Appendix 2 ................................................................................................................... 9 Appendix 3 ................................................................................................................. 12 Appendix 4 ................................................................................................................. 13 Appendix 5 ................................................................................................................. 14 Appendix 6 ................................................................................................................. 15 Appendix 7 ................................................................................................................ -

Windlesham Neighbourhood Plan 2018-2028 Referendum Version
Windlesham Neighbourhood Plan 2018-2028 Referendum Version Windlesham Parish Council The Council Offices, Lightwater Contents INTRODUCTION ................................................................................................................... 3 Foreword from the Parish Council .............................................................................. 3 A Neighbourhood Plan – Defined ............................................................................... 4 How Windlesham benefits from a Plan ...................................................................... 4 Restrictions to the Neighbourhood Plan process ....................................................... 5 How the Plan was prepared ........................................................................................ 6 Submission Plan and Examination .............................................................................. 8 THE DEVELOPMENT OF WINDLESHAM ............................................................................... 9 Growth and Nature ..................................................................................................... 9 THE PRESENT SITUATION .................................................................................................. 10 The Setting ................................................................................................................ 10 Population ................................................................................................................. 10 Employment and Travel -

Durham E-Theses
Durham E-Theses An experiential and comparative analysis of the landscapes of movement and visibility at ve Late Iron Age earthwork complexes in Britain BITHELL, SAMUEL,THOMAS How to cite: BITHELL, SAMUEL,THOMAS (2020) An experiential and comparative analysis of the landscapes of movement and visibility at ve Late Iron Age earthwork complexes in Britain, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/13691/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 An experiential and comparative analysis of the landscapes of movement and visibility at five Late Iron Age earthwork complexes in Britain By Samuel Thomas Bithell Abstract: In recent decades, the territorial oppida of Late Iron Age Britain have begun to be assessed more as landscape constructs than individual sites. In addition, studies of other contemporary complexes frequently excluded from the classification of oppida have revealed remarkable similarities with the traditionally defined territorial oppida. -

Consultation Statement Lightwater Village Design
CONSULTATION STATEMENT LIGHTWATER VILLAGE DESIGN STATEMENT SUPPLEMENTARY PLANNING DOCUMENT Prepared under Regulation 18(4)(b) of the Planning and Compulsory Purchase Act 2004: The Town and Country Planning (Local Development)(England) Regulations 2004. 1. Consultation on the Draft Supplementary Planning Document Before adopting the Draft Lightwater Village Design Statement Supplementary Planning Document (SPD), the Council undertook a six-week consultation exercise on the Draft SPD. This was in accordance with Regulation 17 of the Town and Country Planning (Local Development) (England) Regulations 2004. The Draft SPD was accompanied by a Sustainability Appraisal Report (SAR). The consultation exercise took place between 16 July to 28 August 2007. As part of this consultation exercise, the Council: a) Consulted those organisations and individuals listed in Appendix 1. b) Sent a letter to all the 3,106 residential and commercial properties in Lightwater notifying them where copies of the documents could be read, and inviting them to the exhibition and meeting c) Undertook an exhibition and public meeting about the SPD on 9 August 2007 at All Saints Church Hall, Lightwater. Appendix 2 records the views put forward at the exhibition and meeting. Posters were placed in key locations in the village. d) Publicised the consultation exercise in Heathscene, the Council’s newsletter, in its Summer 2007 edition which was distributed to all residents of the Borough in July 2007. e) Issued a press release about the consultation exercise in the week ending 13 July 2007. f) Published statutory advertisements about the consultation exercise in the Woking News and Mail and the Chobham and Windlesham News and Mail on 12 July 2007 and the Camberley News on 13 July 2007.