Evaluation of Teratogenic Potentials of Bronchodilator Drug on Offsprings of Albino Rats Abd El Wahab El Ghareeb, Hamida Hamdi, EL-Sayed Fahim Taha, Heba Ali
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Theophylline-7-Acetic Acid
Theophylline-7-acetic acid sc-237085 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Theophylline-7-acetic acid STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH2 HAZARD INSTABILITY0 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS C9-H10-N4-O4, "purine-7-acetic acid, 1, 2, 3, 6-tetrahydro-1, 3-dimethyl-2, 6-dioxo-", acefylline, acephylline, 7-(carboxymethyl)theophylline, "1, 2, 3, 6-tetrahydro-1, 3-dimethyl-2, 6-dioxopurine-7-acetic acid", "7-theophyllineacetic acid", "7-theophyllinylacetic acid", alkaloid Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 1 Toxicity: 2 Body Contact: 2 Min/Nil=0 Low=1 Reactivity: 1 Moderate=2 High=3 Chronic: 2 Extreme=4 1 of 8 CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Harmful if swallowed. Irritating to eyes, respiratory system and skin. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ! Accidental ingestion of the material may be harmful; animal experiments indicate that ingestion of less than 150 gram may be fatal or may produce serious damage to the health of the individual. ! Xanthine derivatives may produce nausea, vomiting, anorexia, stomach pain, vomiting of blood and diarrhea. Protein in the urine, increased amounts of urine output, and increased excretion of renal tubular cells and red blood cells may also occur. -

Bahrain-Pharma-1444108170.Pdf
Fill Type of Competition S. No BP Brand Name Therapeutical Class Potency Generic Name/Composition Volume Dosage Name,company, retail price form Each 5 ml contents: Samol 120 ml Salbutamol Sulphate 2mg Salbutamol 1 Anti-Asthmatic agent (Bronchodilator) Each 5ml contains Acefylline piperazine 125mg Pifalin 100 ml Acefylline Piperazine 2 (125mg/5ml) Acefylline Piperazine 45mg, Diphenhydramine Dephicef 100 ml Acefylline Piperazine + Diphenhydramine 3 8mg/5ml Ammonium Chloride 100.00mg , Sodium Clomadrin 100 ml Citrate 60.00mg, Ephedrine HCl 7.00mg, Ammonium Chloride + Sodium Citrate + Ephedrine HCl + Chlorpheniramine Maleate 4 Chlorpheniramine Maleate 2.00mg/5ml Each 5 ml contents: Ambroxol HCl eq. to 100 ml Ambroxol 15mg 5 Ambrol Ambroxol HCl Each 5 ml contents: Ambroxol HCl eq. to Cough and Cold 100 ml Syrup 6 Ambroxol 30mg 7 Dextron 100 ml 15mg/5 ml Dextromethorphan Hbr 8 Gufosil 100 ml 100mg/5 ml Guaifenesin 9 Proligen 120 ml 1.25mg + 30mg/5 ml Triprolodin HCl + Pseudoephedrine HCl 10 Tripodil 120 ml 1.25mg + 30mg + 10mg/5 ml Triprolodin HCL + Pseudoephedrine HCl + Dextromethorphan Hbr 11 Tripogin 120 ml 1.25mg + 30mg + 100 mg/5 ml Triprolodin HCL + Pseudoephedrine HCl + Guaifenesin 12 Dexotrin 120 ml 1.25mg +10mg + 7.5mg + 50mg/5 ml Triprolodin HCL + Pseudoephedrine HCl + Dextromethorphan Hbr + Guaifenesin Wild cherry (Prunus serotina) + (myroxylon balsamum) + Mallow (Malva Sylvestris) + Welcosin 120 ml 60mg + 18.75mg + 7.5mg + 7.5mg / 5ml 13 Marshnallow (Althaea officinalis) 14 Ivosil 100 ml Each 5 ml contents: Ivy leaf 15 Hexobim 100 ml 4mg/5mlIvy leaves dried extract (4-8:1, 100%) 100 mg Bromhexine HCl Carbocisteine 2% and 5% (i.e. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Acefylline Piperazine/Bambuterol Hydrochloride 1115 1 Mg of Aminophylline
Acefylline Piperazine/Bambuterol Hydrochloride 1115 1 mg of aminophylline. The USP 31 specifies that ami- Oral modified-release preparations are given to children with a Preparations nophylline preparations should be labelled with respect body-weight over 40 kg in the long-term management of chronic Proprietary Preparations (details are given in Part 3) bronchospasm. An initial dose of 225 mg twice daily may be Jpn: Solfa; Neth.: Miraftil; USA: Aphthasol. to their anhydrous theophylline content. As the phar- given if the child has not previously received xanthine prepara- macokinetics of theophylline are affected by a number tions, increased after 1 week to 450 mg twice daily according to of factors including age, smoking, disease, diet, and serum-theophylline concentrations. Different modified-release Arformoterol Tartrate (USAN, rINNM) ⊗ drug interactions, the dose of aminophylline must be preparations are not considered interchangeable. carefully individualised and serum-theophylline con- Aminophylline may also be used in the management of neonatal Arformotérol, Tartrate d’; Arformoteroli Tartras; R,R-Formoterol Tartrate; Tartrato de arformoterol. (-)-N-[2-Hydroxy-5-((1R)-1- centrations monitored (see Uses and Administration of apnoea (see p.1118). Although the injection is unlicensed in the UK in children under 6 months of age, the BNFC recommends hydroxy-2-{[(1R)-2-(4-methoxyphenyl)-1-methylethyl]ami- Theophylline, p.1146). an initial dose of 6 mg/kg by intravenous injection over 20 min- no}ethyl)phenyl]formamide hydrogen (2R,3R)-2,3-dihydroxybu- In the management of acute severe bronchospasm, utes. This is followed by 2.5 mg/kg every 12 hours, increased if tanedioate. aminophylline may be given intravenously by slow in- necessary to 3.5 mg/kg every 12 hours. -
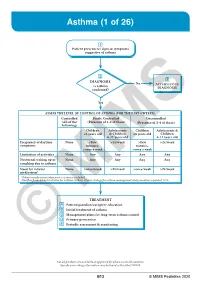
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough -

Acefylline Piperazine/Bambuterol Hydrochloride 1115 1 Mg of Aminophylline
Acefylline Piperazine/Bambuterol Hydrochloride 1115 1 mg of aminophylline. The USP 31 specifies that ami- Oral modified-release preparations are given to children with a Preparations nophylline preparations should be labelled with respect body-weight over 40 kg in the long-term management of chronic Proprietary Preparations (details are given in Part 3) bronchospasm. An initial dose of 225 mg twice daily may be Jpn: Solfa; Neth.: Miraftil; USA: Aphthasol. to their anhydrous theophylline content. As the phar- given if the child has not previously received xanthine prepara- macokinetics of theophylline are affected by a number tions, increased after 1 week to 450 mg twice daily according to of factors including age, smoking, disease, diet, and serum-theophylline concentrations. Different modified-release Arformoterol Tartrate (USAN, rINNM) ⊗ drug interactions, the dose of aminophylline must be preparations are not considered interchangeable. carefully individualised and serum-theophylline con- Aminophylline may also be used in the management of neonatal Arformotérol, Tartrate d’; Arformoteroli Tartras; R,R-Formoterol Tartrate; Tartrato de arformoterol. (-)-N-[2-Hydroxy-5-((1R)-1- centrations monitored (see Uses and Administration of apnoea (see p.1118). Although the injection is unlicensed in the UK in children under 6 months of age, the BNFC recommends hydroxy-2-{[(1R)-2-(4-methoxyphenyl)-1-methylethyl]ami- Theophylline, p.1146). an initial dose of 6 mg/kg by intravenous injection over 20 min- no}ethyl)phenyl]formamide hydrogen (2R,3R)-2,3-dihydroxybu- In the management of acute severe bronchospasm, utes. This is followed by 2.5 mg/kg every 12 hours, increased if tanedioate. aminophylline may be given intravenously by slow in- necessary to 3.5 mg/kg every 12 hours. -

X-Ray Fluorescence Analysis Method Röntgenfluoreszenz-Analyseverfahren Procédé D’Analyse Par Rayons X Fluorescents
(19) & (11) EP 2 084 519 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: G01N 23/223 (2006.01) G01T 1/36 (2006.01) 01.08.2012 Bulletin 2012/31 C12Q 1/00 (2006.01) (21) Application number: 07874491.9 (86) International application number: PCT/US2007/021888 (22) Date of filing: 10.10.2007 (87) International publication number: WO 2008/127291 (23.10.2008 Gazette 2008/43) (54) X-RAY FLUORESCENCE ANALYSIS METHOD RÖNTGENFLUORESZENZ-ANALYSEVERFAHREN PROCÉDÉ D’ANALYSE PAR RAYONS X FLUORESCENTS (84) Designated Contracting States: • BURRELL, Anthony, K. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Los Alamos, NM 87544 (US) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR (74) Representative: Albrecht, Thomas Kraus & Weisert (30) Priority: 10.10.2006 US 850594 P Patent- und Rechtsanwälte Thomas-Wimmer-Ring 15 (43) Date of publication of application: 80539 München (DE) 05.08.2009 Bulletin 2009/32 (56) References cited: (60) Divisional application: JP-A- 2001 289 802 US-A1- 2003 027 129 12164870.3 US-A1- 2003 027 129 US-A1- 2004 004 183 US-A1- 2004 017 884 US-A1- 2004 017 884 (73) Proprietors: US-A1- 2004 093 526 US-A1- 2004 235 059 • Los Alamos National Security, LLC US-A1- 2004 235 059 US-A1- 2005 011 818 Los Alamos, NM 87545 (US) US-A1- 2005 011 818 US-B1- 6 329 209 • Caldera Pharmaceuticals, INC. US-B2- 6 719 147 Los Alamos, NM 87544 (US) • GOLDIN E M ET AL: "Quantitation of antibody (72) Inventors: binding to cell surface antigens by X-ray • BIRNBAUM, Eva, R. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

European Patent Office
Europäisches Patentamt *EP001021204B1* (19) European Patent Office Office européen des brevets (11) EP 1 021 204 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: A61K 47/32, A61L 25/00 of the grant of the patent: 28.12.2005 Bulletin 2005/52 (86) International application number: PCT/US1998/020091 (21) Application number: 98949505.6 (87) International publication number: (22) Date of filing: 25.09.1998 WO 1999/015210 (01.04.1999 Gazette 1999/13) (54) BIOADHESIVE COMPOSITIONS AND METHODS FOR TOPICAL ADMINISTRATION OF ACTIVE AGENTS BIOLOGISCHE KLEBER UND VERFAHREN ZUR TOPISCHEN VERABREICHUNG VON WIRKSTOFFEN COMPOSITIONS BIOADHESIVES ET METHODES D’ADMINISTRATION LOCALE D’AGENTS ACTIFS (84) Designated Contracting States: • HOUZE, David AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Miami, FL 33186 (US) MC NL PT SE • KANIOS, David Miami, FL 33196 (US) (30) Priority: 26.09.1997 US 61155 P (74) Representative: Isenbruck, Günter (43) Date of publication of application: Isenbruck, Bösl, Hörschler, Wichmann, Huhn 26.07.2000 Bulletin 2000/30 Patentanwälte Theodor-Heuss-Anlage 12 (73) Proprietor: NOVEN PHARMACEUTICALS, INC. 68165 Mannheim (DE) Miami, FL 33186 (US) (56) References cited: (72) Inventors: FR-A- 2 532 546 GB-A- 1 050 070 • MANTELLE, Juan GB-A- 2 046 773 US-A- 4 593 053 Miami, FL 33176 (US) US-A- 5 656 286 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data.