List of Designated Food Additives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Fortify Beverages with Calcium by Dr
Ingredients How to Fortify Beverages With Calcium by Dr. Gerhard Gerstner Along with current developments of the overall functional foods market, the use of minerals and especially calcium salts is expected to exhibit strong growth rates. In contrary to other functional ingredients, calcium is widely known as being beneficial for human health and there is no need to explain its nutritional ad- vantages to the customer. According to Leatherhead International, future trends include growing consumer concern regarding osteoporosis and bone health, leading to increased sales of calcium salts. The con- observation is seen as being one of tinuous market growth drives mineral the main factors causing osteo- Common calium sources salt suppliers to offer not only one porosis 2 .As a consequence, national for beverage fortification product but rather a range of different authorities all over the world have calcium salts and granulations to be recently reconsidered recommend- Table 1 shows a typical range of able to tune them to industrial cus- ations in order to take remedial calcium fortified beverages which tomers’ applications. This article measures against calcium deficiency have been seen in European and US discusses important nutritional, and accordingly, to reduce the risk of supermarkets recently. Practically technological as well as economical osteoporosis. In this respect, the US every type of beverage such as aspects of calcium in beverages with National Institute of Health (NIH) has mineral water, soy milk, energy drink, a focus on our company’s products increased the amounts of optimal nectar or juice does have a fortified Tricalcium Citrate, Calcium Gluconate daily calcium intake and defined product line already. -

Calcium Supplements | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Calcium Supplements This information explains calcium supplements and how to take them. Calcium is a mineral that you need to build and maintain healthy bones. If you don’t get enough calcium from your diet, your body will take it from your bones. This can cause osteoporosis. Osteoporosis Osteoporosis develops when you lose bone tissue, which makes your bones more likely to fracture (break). Osteoporosis is most common in females who have gone through menopause (a permanent end of your monthly periods). It can develop in anyone, including males, due to medication or illness. Some risk factors for osteoporosis include: Having a thin build Being of Northern European or Asian descent Having fair skin Going through menopause early (before the age of 45) Taking certain steroid medications for longer than 3 months Calcium Supplements 1/9 Not getting enough physical activity Not getting enough calcium in your diet (or from dietary supplements) Smoking Drinking too much alcohol (more than 2 drinks per day for females or 3 drinks per day for males) Taking aromatase inhibitors (medications that stop the production of estrogen and are used to treat breast cancer) Vitamin D Vitamin D is a vitamin that helps your body absorb calcium. Your body makes vitamin D after being exposed to the sun. Vitamin D is also found in some foods. It can be hard to get enough vitamin D from just sunlight and foods. Your doctor or clinical dietitian nutritionist might tell you to take vitamin D supplements. These can be prescription or over-the-counter vitamin D supplement pills or calcium supplements with vitamin D added. -

HPLC for Food Analysis
HPLC for Food Analysis A Primer © Copyright Agilent Technologies Company, 1996-2001. All rights reserved. Reproduction, adaption, or translation without prior written permission is prohibited, except as allowed under the copyright laws. Printed in Germany www.agilent.com/chem September 01, 2001 Publication Number 5988-3294EN HPLC for Food Analysis A Primer The fundamentals of an alternative approach to solving tomorrow’s measurement challenges Angelika Gratzfeld-Hüsgen and Rainer Schuster Acknowledgements We would like to thank Christine Miller and John Jaskowiak for their contributions to this primer. Mrs. Miller is an application chemist with Agilent Technologies and is responsible for the material contained in chapter 5. Mr. Jaskowiak, who wrote chapter 7, is a product manager for liquid chromatography products at Agilent Technologies. © Copyright Agilent Technologies Company 1996-2001. All rights reserved. Reproduction, adaption, or translation without prior written permission is prohibited, except as allowed under the copyright laws. Printed in Germany, September 1, 2001. Publication Number 5988-3294EN Preface Modern agriculture and food processing often involve the use of chemicals. Some of these chemicals and their func- tions are listed below: • Fertilizers: increase production of agricultural plants • Pesticides: protect crops against weeds and pests • Antibiotics: prevent bacteria growth in animals during breeding • Hormones: accelerate animal growth • Colorants: increase acceptability and appeal of food • Preservatives and antioxidants: extend product life • Natural and artificial sweeteners and flavors: improve the taste of food • Natural and synthetic vitamins: increase the nutritive value of food • Carbohydrates: act as food binders Such chemicals improve productivity and thus increase competitiveness and profit margins. However, if the amounts consumed exceed certain limits, some of these chemicals may prove harmful to humans. -

Laboratory Study of Polychlorinated Biphenyl (PCB) Contamination and Mitigation in Buildings Part 4. Evaluation of the Activate
EPA/600/R-11/156C November 2012 Laboratory Study of Polychlorinated Biphenyl (PCB) Contamination and Mitigation in Buildings Part 4. Evaluation of the Activated Metal Treatment System (AMTS) for On-site Destruction of PCBs Xiaoyu Liu and Zhishi Guo U.S. Environmental Protection Agency Office of Research and Development National Risk Management Research Laboratory Air Pollution Prevention and Control Division Research Triangle Park, NC 27711 and Corey A. Mocka, R. Andy Stinson, Nancy F. Roache, and Joshua A. Nardin ARCADIS, US Inc. 4915 Prospectus Dr., Suite F Durham, NC 27709 NOTICE This document has been reviewed internally and externally in accordance with the U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Executive Summary E.1 Background Polychlorinated biphenyls (PCBs) were once used as a plasticizer in certain building materials such as caulking, sealants, and paints from the 1950s through the late 1970s. Because PCBs have a variety of adverse health effects in animals and human, federal regulations have specific requirements for use and disposal of PCB-containing materials (U.S. EPA, 2005; 2009). Briefly, building materials that contain 50 ppm or more PCBs are not authorized for use and must be disposed of as PCB bulk product waste according the Code of Federal Regulations 40 CFR §761.3 and §761.62. If PCBs have contaminated either the surrounding building materials or adjacent soil, these materials are considered PCB remediation waste, which is subject to the cleanup and disposal requirements according 40 CFR §761.61. -
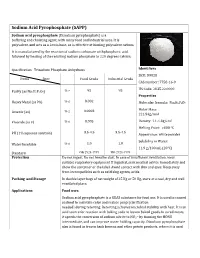
Sodium Acid Pyrophosphate (SAPP)
Sodium Acid Pyrophosphate (SAPP) Sodium acid pyrophosphate (Disodium pyrophosphate) is a buffering and chelating agent, with many food and industrial uses. It is polyvalent, and acts as a Lewis base, so is effective at binding polyvalent cations. It is manufactured by the reaction of sodium carbonate withphosphoric acid. followed by heating of the resulting sodium phosphate to 220 degrees Celsius. Specification: Trisodium Phosphate Anhydrous Identifiers SKU: D9028 Items Spec Food Grade Industrial Grade CAS number: 7758-16-9 HS Code: 2835.22.0000 Purity (as Na2H2P2O7) % ≥ 95 95 Properties % ≤ 0.002 Heavy Metal (as Pb) Molecular formula: Na2H2P2O7 Molar Mass: Arsenic (as) % ≤ 0.0003 221.94g/mol Fluoride (as F) % ≤ 0.005 Density: 1.1-1.3g/cm3 Melting Point: >600 °C 3.5-4.5 3.5-4.5 PH (1% aqueous solution) Appearance: white powder Solubility in Water: Water Insoluble % ≤ 1.0 1.0 11.9 g/100 mL (20°C ) Standard GB 2928-1999 HG 2928-1999 Protection Do not ingest. Do not breathe dust. In case of insufficient ventilation, wear suitable respiratory equipment If ingested, seek medical advice immediately and show the container or the label. Avoid contact with skin and eyes. Keep away from incompatibles such as oxidizing agents, acids. Packing and Storage In double layer bags of net weight of 25 Kg or 50 Kg, store at a cool, dry and well ventilated place. Applications Food uses Sodium acid pyrophosphate is a GRAS substance for food use. It is used in canned seafood to maintain color and reduce purge[clarification needed] during retorting. Retorting achieves microbial stability with heat. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

A Pilot Nursery Study of the Missouri Gravel Bed System On
SUNY College of Environmental Science and Forestry Digital Commons @ ESF Dissertations and Theses Winter 12-11-2017 A PILOT NURSERY STUDY OF THE MISSOURI GRAVEL BED SYSTEM ON PHYTOREMEDIATION TREE SPECIES, POPULUS DELTOIDES X POPULUS NIGRA DN34 AND PINUS NIGRA, FOR POTENTIAL GROWTH ENHANCEMENT OVER SOIL GROWN TREES Thomas Frontera [email protected] Follow this and additional works at: https://digitalcommons.esf.edu/etds Recommended Citation Frontera, Thomas, "A PILOT NURSERY STUDY OF THE MISSOURI GRAVEL BED SYSTEM ON PHYTOREMEDIATION TREE SPECIES, POPULUS DELTOIDES X POPULUS NIGRA DN34 AND PINUS NIGRA, FOR POTENTIAL GROWTH ENHANCEMENT OVER SOIL GROWN TREES" (2017). Dissertations and Theses. 45. https://digitalcommons.esf.edu/etds/45 This Open Access Thesis is brought to you for free and open access by Digital Commons @ ESF. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of Digital Commons @ ESF. For more information, please contact [email protected], [email protected]. A PILOT NURSERY STUDY OF THE MISSOURI GRAVEL BED SYSTEM ON PHYTOREMEDIATION TREE SPECIES, POPULUS DELTOIDES X POPULUS NIGRA DN34 AND PINUS NIGRA, FOR POTENTIAL GROWTH ENHANCEMENT OVER SOIL GROWN TREES by Thomas John Frontera II A thesis submitted in partial fulfillent of the requirements for the Master of Science Degree State University of New York College of Environmental Science and Forestry Syracuse, New York May 2018 Department of Landscape Architecture Approved by: Tim Toland, Major Professor Dr. Lee Newman, Steering Committee Member Dr. Doug Johnston, Steering Committee Member and Department Chair Terry Ettinger, Thesis Examiner Dr. Jose Giner, Examining Committee Chair S. Scott Shannon, Dean, The Graduate School © 2018 Copyright T.J. -

Some Factors Affecting the Level and Persistence of Diphenyl Residues in Citrus Fruits
HAYWARD AND EDWARDS: DIPHENYL RESIDUES 315 Using a drum dryer, temperatures as high as ing the adaptation and use of the double-drum 70° C can be used for densifying orange and dryer. grapefruit powders without discoloration. Mono- glyceride derivatives are effective as release LITERATURE CITED agents during the densifying operation. Recon- 1. Berry, R. E., O. W. Bissett, C. J. Wagner, Jr., and stitution time is increased somewhat by the densi M. K. Veldhuis 1964. Foam-mat dried grapefruit powders. Food Technol. In press. fying operation. 2. Bissett, O. W., J. H. Tatum, C. J. Wagner, Jr., M. K. Veldhuis, R. P. Graham and A. I. Morgan, Jr. 1963. Foam-mat dried orange juice. I. Time-temperature drying studies. Food Technol. 17:92-95. Acknowledgment 3. Graham, R. P., M. R. Hart and A. I. Morgan, Jr. 1964. Foam-mat drying citrus juices. Abstracts of 24th An The authors would like to thank Dr. A. I. nual Meeting of Institute of Food Technologists, No. 29. 4. Graham, R. P., L. F. Ginnette and A. I. Morgan, Jr. Morgan, Jr., and Mr. R. P. Graham of the West 1963. U. S. Patent No. 3,093,488 Preparation of stable de hydrated products. ern Regional Research Laboratory, Albany, Cali 6. Lawler, Frank K. 1962. Foam-mat drying goes to fornia, for their advice and suggestions concern work. Food Eng. 34:68-69. 6. Sjogren, C. N. 1962. Practical facts of foam-mat. Food Eng. 34:44-47. SOME FACTORS AFFECTING THE LEVEL AND PERSISTENCE OF DIPHENYL RESIDUES IN CITRUS FRUITS F. -

Total Diet Study Market Baskets 2004-1 Through 2005-4
U.S Food and Drug Administration – Total Diet Study Market Baskets 2004-1 through 2005-4 The data that follow are summaries of pesticide analytical results by food or by residue from the Food and Drug Administration’s Total Diet Study Program. The information covers Total Diet Study Market Baskets 2004-1 through 2005-4 (8 Market Baskets). The Market Baskets were collected between October 2003 and August 2005. Notes: Food #: FDA assigned Total Diet Study Food number. Number of Analyses: Number of times the food was analyzed for this report (i.e., the number of Market Baskets analyzed containing the food item). Number Equal /> LQ: Number of results for the residue that were greater than or equal to the method’s limit of quantitation. Number of Traces: Number of results for the residue that were equal to or greater than the method’s limit of detection, but less than the method’s limit of quantitation. Statistics were calculated using a value of 0 for results below the method’s limit of detection, and at the measured value for findings recorded as trace. Some values may have been rounded. BF: Baby Food RTF: Ready to Feed The data presented in this report were compiled by FDA’s Kansas City District Laboratory located in Lenexa, KS, with special thanks to Pesticide Expert Chris Sack. Inquiries regarding the data may be referred to: FDA/Center for Food Safety and Applied Nutrition Office of Food Safety 5100 Paint Branch Parkway College Park, MD 20740 Attention: Ronald R. Roy email: [email protected] phone: 240-402-2061 2004-2005 Summary -

US EPA, Pesticide Product Label, PRISTINE FUNGICIDE, 07/08/2014
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION Ms. Christine M. Keating Product Registration Manager mi np 26 Davis Drive JUL U 9 Research Triangle Park, NC 27709-3528 Subject: Pristine Fungicide EPA Reg. No. 7969-199 EPA Decision Number: 492621 Your master and globe artichoke supplemental label submitted on June 17, 2014 to update tank mix instructions; add citrus black spot to citrus diseases; remove California restrictions; and roll in the radicchio supplemental label Dear Ms. Keating: The master label and supplemental label referred to above, submitted in connection with registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), as amended are acceptable. A stamped copy of your labeling is enclosed for your records. This labeling supersedes all previously accepted labeling. You must submit one (1) copy of the final printed labeling before you release the product for shipment with the new labeling. In accordance with 40 CFR 152.r30(c), you may distribute or sell this product under the previously approved labeling for 18 months from the date of this letter. After 18 months, you may only distribute or sell this product if it bears this new revised labeling or subsequently approved labeling. "To distribute or sell" is defined under FIFRA section 2(gg) and its implementing regulation at 40 CFR 152.3. If these conditions are not complied with, the registration will be subject to cancellation in accordance with FIFRA §6(e). Your release for shipment of the product constitutes acceptance of these conditions. One copy of the labels stamped "Accepted" are enclosed for your records. -

Insecticide/Miticide ACTIVE INGREDIENT: by WT. Bifenthrin
RESTRICTED USE PESTICIDE Toxic to fish and aquatic organisms. For retail sale to and use only by certified applicators, or persons under their direct supervision and only for the uses covered by the certified applicator's certification. Insecticide/Miticide ACTIVE INGREDIENT: BY WT. Bifenthrin: (2 methyl[1, 1'-biphenyl]-3-yl) methyl 3- (2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethyl-cyclopropanecarboxylate* . 25.0% OTHER INGREDIENTS**: . 75.0% TOTAL 100.0% *Cis isomers 97% minimum, trans isomers 3% maximum. **Contains petroleum distillates. This product contains 2 pounds active ingredient per gallon. Bifentrhin U.S. Patent No. 4,238,505 KEEP OUT OF REACH OF CHILDREN WARNING—AVISO Si usted no entiende la etiqueta, busque a siguien para que se la explique a usted en detalle. (If you do not understand this label, find someone to explain it to you in detail.) FIRST AID If Swallowed: • Immediately call a poison control center or doctor. • Do not induce vomiting unless told to do so by the poison control center or doctor. • Do not give any liquids to the person. • Do not give anything by mouth to an unconscious person. If In Eyes: • Hold eye open and rinse slowly and gently with water for 15-20 minutes. • Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. • Call a poison control center or doctor for treatment advice. If on Skin • Take off contaminated clothing. or Clothing: • Rinse skin immediately with plenty of water for 15-20 minutes. • Call a poison control center or doctor for treatment advice. -

Effect of Citrus Fruit (Sudachi) Juice on Absorption of Calcium
Food Sci. Technol. Res., +, (+), ,1ῌ-*, ,**0 E#ect of Citrus Fruit (Sudachi) Juice on Absorption of Calcium from Whole Small Fish in Healthy Young Men +ῌ , , + - . Yoshitaka NII , Takamasa OSAWA , Daisuke KUNII , Kazuhiro FUKUTA , Kentaro SAKAI , Maki KONDO and , Shigeru YAMAMOTO + Food Technology Division, Tokushima Prefectural Industrial Technology Center, ++ῌ, Nishibari, Saika-cho, Tokushima 11*ῌ2*,+, Japan , Department of International Public Health Nutrition, Institute of Health Biosciences, The University of Tokushima Graduate School, -ῌ+2ῌ+/ Kuramoto-cho, Tokushima 11*ῌ2/*-, Japan - Department of Nutrition and Health Promotion, Faculty of Human Life Science, Hiroshima Jogakuin University, .ῌ+-ῌ+ Ushita- Higashi, Higashi-ku, Hiroshima 1-,ῌ**0-, Japan . Faculty of Human Life Science, Shikoku University, +,-ῌ+ Ebisuno, Furukawa, Ojin-cho, Tokushima 11+ῌ++3,, Japan Received July +, ,**/; Accepted January ,/, ,**0 Shirasuboshi (boiled and semi-dried whitebait) is a processed seafood that is abundant in calcium. It is eaten whole and commonly consumed in Japan. In this study, we examined the e#ect of Sudachi (Citrus sudachi Hort. ex. Shirai) juice on calcium, magnesium and phosphorus bioavailability in healthy young men. Dried shirasuboshi powder treated with distilled water (C) or sudachi juice (S,*) was prepared for use in two experimental diets, the control diet and the sudachi diet. Either S,* or C was added to a basal diet with a low calcium content (+2* mg/d). The basal diet and the two experimental diets were each consumed for 0 d by six healthy young men according to a randomized and crossover design. The apparent absorp- tion and retention of calcium, magnesium and phosphorus from shirasuboshi were determined and were found to be significantly higher in the sudachi diet than in the control diet.