HBV Cccdna: the Stumbling Block for Treatment of HBV Infection Shousheng Liu and Yongning Xin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aster DM Healthcare Limited 26 August 2019 the Secretary Listing
26 August 2019 The Secretary The Manager, Listing Department, Listing Department, BSE Limited, The National Stock Exchange of India Ltd 1st Floor, Phiroze Jeejeebhoy Towers Exchange Plaza, C-1, Block G Dalal Street, Mumbai 400001 Bandra Kurla Complex Scrip Code: 540975 Bandra (East), Mumbai 400051 Scrip Symbol: ASTERDM Dear Sir/Madam, Sub: Investor Presentation for Capital Market Day With reference to the captioned subject and the announcement made by the Company on August 8, 2019 relating to the Capital Market Day, which was scheduled for August 22, 2019, please find enclosed the Investor Presentation made. Kindly take the above said information on record as per the requirement of SEBI (Listing Obligations and Disclosure Requirements) Regulations, 2015. Thanking You. Yours faithfully For Aster DM Healthcare Limited Puja Aggarwal Company Secretary and Compliance Officer Aster DM Healthcare Limited CIN- L85110KL2008PLC021703 IX/475L, Aster Medcity,Kuttisahib Road Near Kothad Bridge, South Chittoor PO Cheranalloor, Kochi- 682027, Kerala, India Tel: +91 484 6699999, Fax: +91 484 6699862 Email:[email protected] ASTER OM HEALTHCARE Investor and Analyst Meet – 22nd Aug 2019 www.asterdmhealthcare.com1 I I Disclaimer This presentation has been prepared by Aster DM Healthcare Limited (the "Company"), content of which was compiled from sources believed to be reliable for informational purposes only and are based on information regarding the Company and the economic, regulatory, market and other conditions as in effect on the date hereof. Subsequent developments may impact the information contained in this presentation, which neither the Company nor its advisors or representatives are under an obligation to update, revise or affirm. -

Kozhikode Branch
INDIAN ACADEMY OF PAEDIATRICS - KOZHIKODE BRANCH IAP KOZHIKODE Activity of March -2019 IAP Kozhikode Office Bearers President - Secretary - Treasurer - Dr T P Ashraf Dr Nihaz Naha K Dr Vishnu Mohan P T Activity of March -2019 INDIAN ACADEMY OF PAEDIATRICS - KOZHIKODE BRANCH RARE DISEASE DAY OBSERVATION Date: 02-March-2019 On the occasion of rare disease day observation, IAP Kozhikode in association with Department Of Pediatrics, GMC Kozhikode and Lysosomal storage disease support society(LSDSS) conducted awareness program. The program was Inaugurated by Dr. VR Rajendran, Principal, GMC Kozhikode. Hrithunanda, a girl affected with Lysosomal Storage Disease lighted the Lamp. Dr C Sreekumar, Superintendent IMCH, Dr Mohandas Nair Addtl Superintendent, GMC Kozhikode, Dr Ashraf T P, President, IAP Kozhikode, Dr Ajithkumar V T, HOD Pediatrics, GMC Kozhikode, Dr Gireesh S, Addl. Professor, Pediatrics and Dr. Nisha, Pediatric Geneticist, Maulana Hospital, Perinthalmanna felicitated the function.Different aspects of LSD, long term management, challenges and state government initiatives in this field were discussed. Activity of March -2019 INDIAN ACADEMY OF PAEDIATRICS - KOZHIKODE BRANCH SIM WARS SIMULATION COMPETITION AT AIIMS, NEW DELHI Date: 03-March-2019 SIM WARS simulation competition at AIIMS, New Delhi on 3rd March A competition on pediatric intensive care scenarios simulated with a high fidelity *manikin* Sim Baby. 1st time such a competition conducted in India by PedStars & Inspire team. Attended by reputed institutions. Dr Manjusha.K,Senior Resident IMCH Kozhikode, Dr Nabeel Faisal.V, Dr Nikhil. K . Sunil and Dr Jasir Usman, mentored by Dr.Jayakrishnan. M. P, Additional Professor, Department of Pediatrics, GMC Kozhikode and and Dr. -

Aster DM Healthcare Limited June 23, 2020 the Secretary Listing
June 23, 2020 The Secretary The Manager, Listing Department, Listing Department, BSE Limited, The National Stock Exchange of India Ltd 1st Floor, Phiroze Jeejeebhoy Towers Exchange Plaza, C-1, Block G Dalal Street, Mumbai 400001 Bandra Kurla Complex Scrip Code: 540975 Bandra (East), Mumbai 400051 Scrip Symbol: ASTERDM Dear Sir/Madam, Sub: Revised Investor Presentation for the quarter and year ended March 31, 2020 With reference to the captioned subject, please find enclosed the revised Investor Presentation on the Company’s performance for the quarter and year ended March 31, 2020. Due to technical distortion while converting the document to machine readable format the slides in the presentation are not appearing clearly. We request you to kindly take the above said information on record as per the requirement of SEBI (Listing Obligations and Disclosure Requirements) Regulations, 2015. Thanking You. Yours faithfully For Aster DM Healthcare Limited Puja Aggarwal Company Secretary and Compliance Officer Aster DM Healthcare Limited CIN- L85110KL2008PLC021703 IX/475L, Aster Medcity,Kuttisahib Road Near Kothad Bridge, South Chittoor PO Cheranalloor, Kochi- 682027, Kerala, India Tel: +91 484 6699999, Fax: +91 484 6699862 Email:[email protected] Investor Presentation – For the quarter ended 31st March 2020 1 Disclaimer ❑ This presentation has been prepared by Aster DM Healthcare Limited (the "Company"), content of which was compiled from sources believed to be reliable for informational purposes only and are based on information regarding the Company and the economic, regulatory, market and other conditions as in effect on the date hereof. Subsequent developments may impact the information contained in this presentation, which neither the Company nor its advisors or representatives are under an obligation to update, revise or affirm. -

Cytopathology & Histopathology
Mahsheena KM, J Cytol Histol 2018, Volume 9 DOI: 10.4172/2157-7099-C1-014 4th International Conference on Cytopathology & Histopathology August 29-30, 2018 | Boston, USA The validity of immunocytochemistry in differentiating between benign and malignant thyroid neoplasms pre-operatively Mahsheena KM Aster MIMS hospital, India Preface: FNAC is the most commonly used diagnostic modality for the pre-operative assessment of thyroid neoplasms. However, FNAC, when used alone, has its own pitfalls including the inability to differentiate between benign and malignant follicular neoplasms, difficulty in identifying a follicular variant of papillary carcinoma etc. This study addresses the issue of whether preferential overexpression of galectin-3 in thyroid malignancies can serve to make a presurgical diagnosis between benign and malignant thyroid neoplasms on FNAC. The other marker used was TTF-1, to identify the thyroid origin of the neoplastic cells. Objective: To estimate the sensitivity, specificity and predictive values of galectin-3 and TTF-1 in detecting malignant neoplasms of the thyroid on FNA smears. Study Sample: FNA smears of solitary thyroid nodules taken at the Cytopathology division in the Department of Pathology, Aster MIMS hospital Calicut a tertiary health care system were analyzed from June 2016 to July 2017. Study Method: Immunocytochemistry was performed with galectin-3 and TTF-1 on fresh FNA smears by the standard protocol. Positive staining for galectin-3 was considered when any single epithelial cell showed cytoplasmic or nucleo- cytoplasmic immunostaining. Positive staining for TTF-1 was considered when any single epithelial cell showed nuclear immunostaining. Postoperative histological diagnosis represented the gold-standard. Results: Out of the total 39 cases Gal-3 positivity was seen in 100% (16/16) cases of malignancy and 9% (2/22) cases of benign thyroid lesions. -

JMSCR Vol||04||Issue||07||Page 11304-11309||July 2016
JMSCR Vol||04||Issue||07||Page 11304-11309||July 2016 www.jmscr.igmpublication.org Impact Factor 5.244 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: http://dx.doi.org/10.18535/jmscr/v4i7.20 105 Cases of Macrocytosis- Etiologic Profile Reflecting Lifestyle Changes Authors Dr M.C.Savithri1, Dr K.P.Kavitha2 1Associate Professor, Dept of Pathology, Amala Institute of Medical Sciences, Thrissur, Kerala. India 2Senior Consultant, Dept of Pathology, Aster MIMS Hospital, Kozhikode, Kerala, India Introduction received in the Department of Pathology for Macrocytosis is encountered frequently during peripheral smear examination were selected for medical practice. Megaloblastic anemia (MA) due this study spanning a period of two years, from to Vitamin B12 or folate deficiency is the well April 2010 to end of March 2012. RBC indices known cause of macrocytosis. Pernicious anemia were obtained using using 5 part electronic is a major cause of megaloblastosis in Western hematology cell counters which were regularly world where their diet supplies the necessary calibrated and maintained. nutrients.[1] Nutritional megaloblastic anemia is Case histories, physical examination findings and very important in the Indian scenario.[2]However details of relevant investigations were retrieved non megaloblastic macrocytosis also forms an from Medical Records Section. There were 137 important group which includes alcoholic liver cases of macrocytosis. Those cases for which no disease, hemolytic anemias, leukemias etc. Kerala further details were available were excluded and is a an Indian State where literacy rate is high. The 105 cases selected. rural urban divide is much less pronounced here Retrospective studies have certain inherent and medical facilities are available even in the problems. -

NEWSLETTER August New.Cdr
Vol. 7 | July 2019 I N D I A N A C A D E M Y O F P E D I A T R I C S K E R A L A IAP MONTHLY E-BULLETIN OF IAP TKERALA DPRIVATE CIARCULATIOYN ONLY OFFICE BEARERS IAP KERALA Dr Santosh M K Dr Balachandar D Dr Narayanan M Dr Jayakumar PR President Secretary President Elect Vice President Dr Krishna Mohan R Dr Johny Sebasan Dr Riaz I Dr Vijayakumar M Treasurer Joint Secretary President's nominee Editor – Companion Dr Ranjith P Dr Vishnu Mohan P T Web Editor Editor - IAP today CENTRAL IAP PAST PRESIDENTS CENTRAL IAP Dr Remesh Kumar R Dr Abraham K Paul Dr MKC Nair Dr T U Sukumaran Honorary Secretary General EB Member President CIAP - 2004 President CIAP 2011 Dr S Sachidananda Kamath Dr PAM Kunju Dr Shimmy Paulose President CIAP 2015 EB Member EB Member President's Message We are really really happy to present July issue of IAP TODAY, monthly news leer of IAP Kerala, because this contains reports from all our 22 branches . Editors and all office bearers are doing excellent work. The acon plans PIVOT is in full swing. We have given maximum importance to Vamping dormant and semi dormant branches and we have succeeded fully in this venture. "Doctors day" was celebrated in great enthusiasm by all branches. 4th EB was in cochin, one of the most effecve EB. Immunisaon workshops conducted by almost all branches in kerala so also Central IAP Modules. We were in media almost everyday in july. -

About Aster DM Healthcare
FY2018 FY18 Revenue up by 13% Y-O-Y; EBITDA up by 79%; PAT up by 189% In FY18 operationalized two new hospitals in GCC • Medcare hospital – Sharjah, UAE (124 beds) • Aster Hospital – Doha, Qatar (61beds) Bangalore, May 21, 2018: Aster DM Healthcare, one of the largest private healthcare service providers in multiple GCC states and an emerging healthcare player in India, today announced its financial results for the quarter and full year ended March 31, 2018. Aster DM Healthcare is a 30-year-old integrated and comprehensive healthcare service organization with presence in 9 countries. The Company is one of the few entities across the globe providing the complete circle of care from primary, secondary, tertiary to quaternary medical care through its 19 hospitals, 101 clinics and over 200 pharmacies. These are manned by its17,335 employees from across the world, delivering on a simple yet strong promise to its people: “We’ll treat you well.” The Company has the unique distinction of serving people by providing quality healthcare to all segments of the society regardless of their economic or social positioning. In line with this, Dr. Azad Moopen, Founder Chairman and Managing Director at Aster DM Healthcare conceptualized the Company’s three brands - Medcare for the high income, Aster for the middle-income and Access for low- income strata of the population. The Company has an asset light business model wherein the land and civil structure of most of its hospitals are leased. It is also optimally positioned in the Medical tourism sector with a large number of GCC residents visiting India to avail quality and cost effective healthcare. -
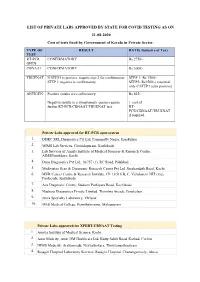
LIST of PRIVATE LABS APPROVED by STATE for COVID TESTING AS on 21-08-2020 Cost of Tests Fixed by Government of Kerala in Private Sector
LIST OF PRIVATE LABS APPROVED BY STATE FOR COVID TESTING AS ON 21-08-2020 Cost of tests fixed by Government of Kerala in Private Sector. TYPE OF RESULT RATE( Inclusive of Tax) TEST RT-PCR CONFIRMATORY Rs 2750/- OPEN CBNAAT CONFIRMATORY Rs 3000/- TRUENAT If STEP1 is positive, require step 2 for confirmation STEP 1- Rs 1500/- STEP 1 negative is confirmatory STEP2- Rs1500/-( required only if STEP1 turns positive) ANTIGEN Positive results are confirmatory. Rs 625/- Negative results in a symptomatic person require + cost of further RT-PCR/CBNAAT/TRUENAT test RT- PCR/CBNAAT/TRUENAT if required Private Labs approved for RT-PCR open system 1. DDRC SRL Diagnostics Pvt Ltd, Panampilly Nagar, Ernakulam 2. MIMS Lab Services, Govindapuram, Kozhikode 3. Lab Services of Amrita Institute of Medical Sciences & Research Centre, AIMSPonekkara, Kochi 4. Dane Diagnostics Pvt Ltd, 18/757 (1), RC Road, Palakkad 5. Medivision Scan & Diagnostic Research Centre Pvt Ltd, Sreekandath Road, Kochi 6. MVR Cancer Centre & Research Institute, CP 13/516 B, C, Vellalaserri NIT (via), Poolacode, Kozhikode 7. Aza Diagnostic Centre, Stadium Puthiyara Road, Kozhikode 8. Neuberg Diagnostics Private Limited, Thombra Arcade, Ernakulam 9. Jeeva Specialty Laboratory, Thrissur 10. MES Medical College, Perinthalmanna, Malappuram Private Labs approved for XPERT/CBNAAT Testing 1. Amrita Institute of Medical Science, Kochi 2. Aster Medcity, Aster DM Healthcare Ltd, Kutty Sahib Road, Kothad, Cochin 3. NIMS Medicity, Aralumoodu, Neyyattinkara, Thiruvananthapuram 4. Rajagiri Hospital Laboratory Services, Rajagiri Hospital, Chunangamvely, Aluva 5. Micro Health LAbs, MPS Tower, Kozhikode 6. Believers Church Medical College Laboratory, St Thomas Nagar, Kuttapuzha P.O., Thiruvalla 7. -

The Nabh Hospital Report by Digit About Digit
THE NABH HOSPITAL REPORT BY DIGIT ABOUT DIGIT Recognized as the General Insurance Company of the Year in Asia by the 23rd Asia Insurance Review Awards 2019 held in Singapore, Digit is one of the world's fastest-growing companies in the world, on a mission to make insurance simple for people. Right from buying an insurance to making claims is quick, hassle-free and can easily be done online without any of the complex paperwork. Our core philosophy is to be simple, and to only make products we would buy for our loved ones too. Everything we do and offer is based on this very philosophy. After all, insurance was meant to make our lives easier and not the other way around. HEALTH INSURANCE BY DIGIT Health is one of the most important things in our lives. That's why, while designing our health insurance product- we decided to seek help from the ones who understand this the best- our health experts a.k.a doctors! With years of experience in healthcare, doctors know exactly the kind of health benefits people may find useful in different phases of their life. This is where our health insurance ticks. With health insurance plans customized by doctors, we help you pick just the right health insurance plan to suit YOU. WHAT’S GREAT ABOUT A HEALTH INSURANCE BY DIGIT? Free Annual Health Check-up No Claim Bonus at renewal up to 100%* Additional Sum Insured for Accidental 2X Sum Insured & Critical Illness Hospitalization Available through Refill Benefit Choose any Hospital Room Type Zero co-payment* with No Restriction on Room Rent* *Condition Apply. -

Health Insurance Tpa of India Ltd—Approved List of Hospitals As on 31.05.2018
HEALTH INSURANCE TPA OF INDIA LTD—APPROVED LIST OF HOSPITALS AS ON 31.05.2018 SR Hospital Name Address City Pin Code State PhoneNo Identifier 1 Aakash Hospital 90/43, Malviya Nagar New Delhi 110017 Delhi 011-40501000 PPN 25 AB, Community Centre, Aashlok Fortis Hospital Safdarjung Enclave, New Delhi, New Delhi 110029 Delhi 011 4616-5901 PPN 2 Delhi 110029 Aditya Varma Medical Centre/ 32, Chitra Vihar New Delhi 110092 Delhi 011-22448008 PPN 3 Aarogya Hospital 011-26680397, Agrawal Eye Institute A - 235,Shivalik,Malviya Nagar New Delhi 110017 Delhi PPN 4 26682203 5 Amar Leela Hospital Pvt. Ltd B - 1/6 Janak Puri New Delhi 110058 Delhi 011-25591345 PPN A-3, Manak Vihar Ext. Near Amit Nursing Home New Delhi 110018 Delhi 011 28122149 PPN 6 Beriwala Bagh,Tihar,New Delhi Plot No-14, Sector-20, Dwarka, Artemis Hospital New Delhi 110075 Delhi 011 7111 1000 PPN 7 New Delhi 8 Ayushman Hospital Plot No.2, Sector - 12,Dwarka New Delhi 110075 Delhi 011 4703 1100 PPN 9 B L Kapur Memorial Hospital Pusa Road New Delhi 110005 Delhi 011-30653019 PPN B.M Gupta Nursing Home H-11-15 Arya Samaj New Delhi 110059 Delhi 011-47157728 PPN 10 Pvt.Ltd. Road,Uttam Nagar 101, Vikas Surya Plaza, 7 D.D.A. Bajaj Eye Care Centre Community Centre Road No.44, New Delhi 110034 Delhi 011-27012054 PPN 11 Pitampura Balaji Medical & Diagnostic 108-A I.P. New Delhi 110091 Delhi 011-43033333 PPN 12 Research Centre (Max Group) Extension,Patparganj 20-B/3, D.B. -

10 Holmium Laser Enucleation of Prostate
Podium Paper ID Paper Title Abstract Author Names 10 HOLMIUM LASER Introduction and Objective To report our experience with initial 500 cases of Holmium Krishna Mohan Ramaswami*, Suzicon; Harigovind p, Aster ENUCLEATION OF PROSTATE Laser Enucleation of Prostate (HoLEP) for BPH and techniques which we adopted to MIMS; Khurshid ahamed, Aster MIMS (HOLEP) : THE CRUCIAL decrease the learning curve. Methods A prospective study was done including all patients TECHNICAL ASPECTS TO who underwent HoLEP at our institution from December 2014 to march 2016. Follow up DECREASE THE LEARNING period ranged from 1 month to 13 months. A pulsed high power 100Watts Holmium Laser CURVE, OUR INSTITUTIONAL with 24F resectoscope sheath was used. Two-lobe or Three -lobe techniques of EXPERIENCE OF 500 CASES. enucleation followed. All patients were preoperatively assessed with ultrasound prostate volume estimation, maximum urine flow rate (Q Max), post void residual urine volume (PVR) assessment and International Prostate Symptom Score(IPSS). Results Patients mean age was 66±8.1 yrs; mean prostate volume was 62±34 cc. Enucleation time was 55 ±22.9 min and morcellation time 15.3±10min, resected weight was 40±27.5g. Catheter time was 36 ± 14.7h and hospital stay was 48±26h. Mean serum hemoglobin and sodium did not drop significantly from baseline after the procedure (p=013).6 patients had extraperitoneal extravasation. A significant improvement occurred in Qmax (25.1±10.7ml/s), PVR and IPSS (0.7±1.3) at follow-up compared with baseline (p<0.05). 11 3D STANDARD LAPAROSCOPIC ABSTRACT With the increase in laparoscopic experience and new instruments natural Krishna Mohan Ramaswami*, Suzicon; Harigovind p, Aster DONOR NEPHRECTOMY orifice vagina has been used to retrieve the donor kidney in selected females called trans MIMS; Khurshid ahamed, Aster MIMS VERSUS TRANSVAGINAL vaginal natural orifice assisted laparoscopic donor nephrectomy (TVNALDN). -

Lack of CPR Knowledge Among Young Medical Doctors: a Worldwide Issue” Karapparambil Vineeth Chandran1, Siju V Abraham2
LETTER TO THE EDITOR In Response to the Letter to the Editor Submitted Titled “Lack of CPR Knowledge among Young Medical Doctors: A Worldwide Issue” Karapparambil Vineeth Chandran1, Siju V Abraham2 Keywords: Basic life support, Cardiopulmonary resuscitation (CPR), Young doctors. Indian Journal of Critical Care Medicine (2021): 10.5005/jp-journals-10071-23697 Dear editor, 1Department of Emergency Medicine, Aster MIMS, Kozhikode, Kerala, This letter is in response to the letter to the editor submitted India titled “lack of CPR knowledge among young medical doctors: a 2Emergency Medicine, Jubilee Mission Medical College and Research worldwide issue.” Institute, Thrissur, Kerala, India It is encouraging to observe that globally researchers are Corresponding Author: Karapparambil Vineeth Chandran, thinking in the same line as we are. It is also interesting to note that Department of Emergency Medicine, Aster MIMS, Kozhikode, Kerala, the results in our study are comparable to those by the European India, Phone: +91 8281282351, e-mail: vineethchandran577@gmail. group.1 com In the light of new discussions, I would like to highlight few How to cite this article: Chandran KV, Abraham SV. In Response to the points from our study2 Letter to the Editor Submitted Titled “Lack of CPR Knowledge among • As rightly pointed out in the letter, the study group consisted Young Medical Doctors: A Worldwide Issue”. Indian J Crit Care Med of a group of doctors who will be exiting the medical school 2021;25(1):107. and joining to work in the community within few weeks’ Source of support: Nil time. Most of them will enroll in some departments in the Conflict of interest: None hospital like emergency medicine, general medicine, general surgery, ICU, and so on to work as junior doctors.