La Difusió D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

February 2017 Monthly Report
Bank of America Credit Card Statement for the Period ending February 28, 2017 TRANSACTION REPORTS TO INTERMEDIATE MERCHANT NAME AMOUNT POSTING DATE COST ALLOCATION - EXPENSE OBJECT EXPENSE DESCRIPTION 311 CENTER SNAPENGAGE CHAT $ 49.00 02/15/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 MONTHLY LIVE CHAT FEE 311 CENTER WPY ONEREACH $ 198.00 02/17/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 MONTHLY LIVE TEXT FEE 311 CENTER AMAZON MKTPLACE PMTS $ 69.30 02/27/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 ANNIVERSARY GIFTS 311 CENTER AMAZON MKTPLACE PMTS $ 99.90 02/27/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 ANNIVERSARY GIFTS 311 CENTER BENNISONS BAKERY INC $ 64.86 02/27/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 OPEN HOUSE 311 CENTER VALLI PRODUCE $ 10.98 02/28/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 ANNIVERSARY PARTY DRINKS 311 CENTER DOLLARTREE $ 7.00 02/28/2017 64505 TELECOMMUNICATIONS CARRIER LINE CH 311 ANNIVERSARY PARTY ITEMS ADMIN SVCS/ FACILITIES ABLE DISTRIBUTORS $ 10.69 02/01/2017 65050 BUILDING MAINTENANCE MATERIAL COVER FOR THERMOSTAT ADMIN SVCS/ FACILITIES THE HOME DEPOT #1902 $ 153.64 02/01/2017 65050 BUILDING MAINTENANCE MATERIAL HEATER PARTS ADMIN SVCS/ FACILITIES CONNEXION $ 100.97 02/01/2017 65050 BUILDING MAINTENANCE MATERIAL OUTDOOR LIGHTS ADMIN SVCS/ FACILITIES THE HOME DEPOT #1902 $ 5.98 02/01/2017 65085 MINOR EQUIP & TOOLS TOOLS ADMIN SVCS/ FACILITIES SHERWIN WILLIAMS 70370 $ 210.56 02/02/2017 65050 BUILDING MAINTENANCE MATERIAL BATHROOM REMODEL PAINT ADMIN SVCS/ FACILITIES RIXON -

British Engine Boiler Insurance Company Limited
British Engine, Boiler and Electrical Insurance Company Limited, 1878- 1991 Location and extent of archival material: SS4/2-8, 57 boxes The listing has been completed in box number order and there has been no attempt to arrange material into series as the collection simply includes a large selection of miscellaneous company catalogues. Company history The Company was founded in Manchester on November 12 1878 under the title of ‘The Engine and Boiler Insurance Company’ with RB Longridge as Chairman and Micheal Longridge (his nephew) as Chief Engineer. The Longridge family were established engineers at the beginning of the 19th Century. They were originally iron masters of Bedlington, Northumberland who also supplied castings and forgings to the engineers of the day. They were involved in the early development of industrial steam engines and were business partners with both George and Robert Stephenson. Members of the Longridge family continued to serve the Insurance Company from the early days until the introduction of nuclear power. RB Longridge formed The Steam Boiler Assurance Company in 1859 but later resigned. Following his resignation he formed ‘The Engine and Boiler Insurance Company Limited’. Within two years the name changed to ‘The Engine, Boiler and Employers’ Liability Company Limited’. In 1903, the Silver Jubilee year the title ‘British Engine, Boiler and Electrical Insurance Company Limited’ was adopted, and in 1978 the title was abbreviated to ‘British Engine Insurance Limited’. The family association terminated in 1966 with the retirement of HM Longridge. The name of Longridge was perpetuated in the title of the Head Office building in Manchester- Longridge House, erected in 1959 on a site embracing that of the original Manchester Steam Users Association of which RB Longridge was Chief Inspector. -

Solar Solutions Bristol Stickney, Technical Director, Cedar Mountain Solar Systems, Santa Fe, N.M
Solar Solutions Bristol Stickney, technical director, Cedar Mountain Solar Systems, Santa Fe, N.M. Bristol’s six principles of good solar hydronic design Basic Combi-System Controls In this series of articles, I have been making the case that the key become my basic standard piping arrangement for over a decade for ingredients for solar/hydronic design and installation can be divided Combi-system 101. There are a number of additional benefits when into six categories, listed below, roughly in order of their importance. using this system in real-world installations. The primary loop is typ- 1. RELIABILITY ically installed horizontally, hanging from the ceiling. The swing 2. EFFECTIVENESS check valves must me installed horizontally or “flow upward,” since 3. COMPATIBILITY they may not close properly if installed “flow downward.” 4. ELEGANCE For the purpose of this discussion, I will present the very simplest 5. SERVICEABILITY control system that will provide the homeowner with Reliable and 6. EFFICIENCY Effective use of the solar heat available from this sample heating The success of any solar hydronic home heating installation system. Keep in mind, that as a simple example, it does not include depends on the often-conflicting balance between any of these six night cooling, baseboard heat, heat storage tanks for space heating principles. Finding the balance between them defines the art of solar or other relatively common options. Each additional option would heating design. require additional control components to be included in our wiring n previous columns I have introduced and explained some of the and control system. So to keep this article brief we will limit the dis- elemental concepts of hydronic solar home heating. -

Supertherm (VS Series) Indirect Fired, Semi-Instantaneous, Steam and Boiler Water Heater (Vertically Built, Steam to Water with Electronic Or Pneumatic Controls)
Installation, Operation, and Maintenance Manual SuperTherm (VS Series) Indirect Fired, Semi-Instantaneous, Steam and Boiler Water Heater (Vertically Built, Steam to Water with Electronic or Pneumatic Controls) This manual applies to the following models: SuperTherm VS06_S2A SuperTherm VS08_S2A SuperTherm VS010_S2A SuperTherm VS012_S2A SuperTherm VS06_D2A SuperTherm VS08_D2A SuperTherm VS010_D2A SuperTherm VS012_D2A CONTRACTOR / FACILITY INFORMATION NOTE: This user manual must always accompany the specific unit as recorded below: Model #: _______________________________ Serial #: _______________________________ Install Date: ____________________________ DHT SUPERTHERM WATER HEATER Latest Update: 09/05/2018 DISCLAIMER The information contained in this document is subject to change without notice from Diversified Heat Transfer, Inc. (DHT). DHT makes no warranty of any kind in respect to this material, including, but not limited to, implied warranties of merchantability and fitness for a particular application. DHT is not liable for errors appearing in this document, nor for incidental or consequential damages occurring in connection with the furnishing, performance, or use of these materials. REV-3 Diversified Heat Transfer, Inc. 439 Main Road, Route 202 Towaco, New Jersey 07082 Page 1 of 90 SuperTherm Manual Phone: 800-221-1522 Website: www.dhtnet.com 02/24/2018 SUPERTHERM Install, Operation, and Maintenance Manual TABLE OF CONTENTS TABLE OF CONTENTS: SECTION 1: GENERAL INFORMATION ................................................................................................... -

Installation and Operation Manual for Solar Indirect Water Heaters
To the Installer: To the Consumer: Please attach these instructions Please read these and all component next to the water heater. instructions and keep for future reference. Solar Indirect Water Heater Instruction Manual Warranty, Registration Card and Parts List are included. Homeowner: Please remember to return the Registration Card! WARNING INSTALLER RESPONSIBILITIES Improper installation, adjustment, alteration, Please read all instructions thoroughly before service or maintenance can cause serious installing or placing the heater into service. injury or property damage. Refer to this This unit must be installed by licensed or manual. For assistance or additional authorized installers, or technical personnel information, consult a qualified installer or that service water heating equipment. The service agency. heater must be installed in accordance with all local codes and ordinances. CAUTION The recommended temperature for normal These instructions are a guide for the correct residential use is 120°F. The dial on the installation of the water heater. The aquastat does not always reflect the out- manufacturer will not be liable for damages coming water temperature, which could caused by failure to comply with the installation occasionally exceed 120°F. The variation in out- and operating instructions outlined on the coming temperature could be based on factors following pages. including but not limited to usage patterns and CAUTION type of installation. Test your water at the tap The recommended water temperature setting nearest to the water heater. for normal residential use is 120°F/49°C. WARNING HANDLING Hotter water increases the risk of scald injury. Before uncrating, check for shipping damage. Before adjusting the water temperature setting, Report any damage to your carrier. -

Idronics 6: Solar Thermal Combisystems
Idronics#6 Feat_rev2.indd 1 5/4/10 2:57:56 PM CaleffiCaleffi North North America, America, Inc. Inc. 98503883 South W. Milwaukee 54th Street Rd Franklin,Milwaukee, WI 53132 Wisconsin 53208 T:T: 414.421.1000414.238.2360 F: F: 414.421.2878 414.238.2366 Dear Hydronic Professional, Dear Hydronic Professional, Welcome to the 6th edition of idronics, Caleffi ’s semi-annual design journal Welcomefor hydronic to theprofessionals. 2nd edition of idronics – Caleffi’s semi-annual design journal for hydronic professionals. The global recession has slowed nearly all industries over the past year. TheHowever, 1st edition despite of idronicsdeclines was in construction, released in January solar water 2007 heater and distributed shipments to over 80,000in the US people increased in North 50% America. during 2008.It focused on the topic hydraulic separation. From the feedback received, it’s evident we attained our goal of explaining the benefits andAs more proper and application more HVAC of professionalsthis modern design become technique familiar forwith hydronic solar water systems. heating, many recognize opportunities to extend solar thermal technology A Technical Journal Iffor you combined haven’t domesticyet received hot a water copy andof idronics space heating#1, you applications.can do so by Thissending is a in the attachedtrend our reader parent response Caleffi SpA card, identifi or by ed registering several years online ago at in www.caleffi.us Europe, and is. The from publicationnow a topic willof growing be mailed interest to you here free inof North charge. America. You can For also this download reason, the Caleffi Hydronic Solutions completesolar “combisystems” journal as a PDF was fileselected from our as theWeb topic site. -

Best Practices Manual for Solar Hot Water Systems
Best Practices Manual for Solar Hot Water Systems Updated: 10-25-2011 Introduction: This manual was developed as a tool to assist solar thermal designers and installers as a guideline to provide the most reliable solar hot-water systems possible. The material presented here is not intended to be used as a list of system requirements or as a type of solar code. Rather, it was assembled with the input of many parties to share lessons learned in the field. It is not inclusive and is a work in progress. This manual was developed in Wisconsin where some parts of the state have over 10,000 heating degree days and where winter temperatures regularly fall below -30°F. In fact, the record coldest temperature recorded in Wisconsin was - 55°F. During the summer, temperatures can rise above 100°F. While most climates are not this severe, the practices outlined in this manual will be helpful for system designs in all cold climates as well as in warm climates. A properly designed solar hot-water system must not only function properly during extreme cold and hot environmental circumstances, it must also be able to safely endure sustained periods of low or no hot water draw without damage or overheating. A best practice is defined as: • A practice that is most appropriate under the circumstances. • A technique or methodology that, through experience and research, has reliably led to a desired or optimum result. Overview: A well-designed solar water heating system that is appropriate for the climate where it is located and is properly installed with appropriate solar rated components will last for many years. -

Leisure Line WL110 Boiler Operations & Maintenance Manual and Safety
Thank you for Purchasing a Leisure Line Coal Stoker Boiler. Proper Care and Maintenance are an important part of getting the most out of your boiler. CAUTION Burn Rice or Buckwheat Sized Anthracite Coal only in the WL110 Boiler. Page 1 of 18 Leisure Line WL110 Boiler Operations & Maintenance Manual And Safety Instructions SAFETY NOTICE PLEASE READ ENTIRE MANUAL BEFORE INSTALLING YOUR NEW BOILER. FAILURE TO FOLLOW THESE INSTRUCTIONS MAY RESULT IN PROPERTY DAMAGE, BODILY INJURY, OR EVEN DEATH CONTACT YOU LOCAL BUILDING AND FIRE OFFICIALS ABOUT RESTRICTIONS AND PERMIT/INSPECTION REQUIREMENTS IN YOUR AREA Black Rock Manufacturing DBA Leisure Line Stove Company Rear 620 Broad Street Berwick, PA 18603 http://www.leisurelinestoves.co m [email protected] [email protected] 570-752-1811 Thank you for Purchasing a Leisure Line Coal Stoker Boiler. Proper Care and Maintenance are an important part of getting the most out of your boiler. CAUTION Burn Rice or Buckwheat Sized Anthracite Coal only in the WL110 Boiler. Page 2 of 18 Contents Important Warning Statements ......................................................................................................................................... 2 General Carbon Monoxide Information, Warning and Caution .................................................................................... 3 Boiler Concept of Operation and General Information ................................................................................................... 4 Unpack and Installation instructions -
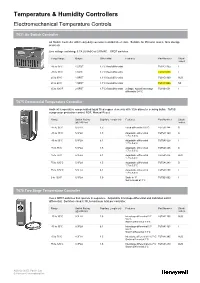
Temperature & Humidity Controllers
Temperature & Humidity Controllers Electromechanical Temperature Controls T631 Air Switch Controller Air Switch Controller with heavy-duty corrosion resistant steel case. Suitable for lift motor rooms, farm storage areas etc. Line voltage switching: 3.7A (I) 5A(R) at 240VAC. SPDT switches Temp Range OutputDifferential FeaturesPart Number Stock/ Indent -35 to 35°C 1 SPDT 1.7°C fixed differential T631C1152 I -10 to 30°C 1 SPDT 1.7°C fixed differential T631C1038 I 20 to 60°C 1 SPDT 1.1°C fixed differential T631C1160 AUS 20 to 60°C 1 SPDT 1.1°C fixed differential T631C1046 NZ 35 to 100°F 2 SPDT1.7°C fixed differential 2 Stage. Adjust' interstage T631B1054 I differential 0-4°C T675 Commercial Temperature Controller Ambient temperature compensated liquid filled copper elements with 1/2in diameter sensing bulbs. T675B compressor protection control, BOF, Manual Reset Range Switch Rating Capillary Length (m) FeaturesPart Number Stock/ (@ 240Vac) Indent -15 to 35°C 0.5FLA1.5 Fixed differential 0.6°CT675A1284 S -15 to 35°C 5.1FLA1.5 Adjustable differential T675A1169 S 1.7 to 5.6°C -15 to 35°C 5.1FLA6.1 Adjustable differential T675A1920 I 1.7 to 5.6°C 15 to 75°C 5.1FLA1.5 Adjustable differential T675A1466 S 1.7 to 5.6°C 15 to 75°C 5.1FLA6.1 Adjustable differential T675A1474 AUS 1.7 to 5.6°C 75 to 125°C 5.1FLA1.5 Adjustable differential T675A1243 S 1.7 to 5.6°C 75 to 125°C 5.1FLA6.1 Adjustable differential T675A1250 I 1.7 to 5.6°C 0 to 100°F 5.1FLA3.0 Scale in °F T675B1002 I Set to break at 3°C T678 Two Stage Temperature Controller Has 2 SPDT switches that operate in sequence. -

Basic Oil Heat Plan -- $309.95 Plus Tax
DANBURY (203) 744-0600 Mitchell BEDFORD HILLS (914) 666-8400 NEW MILFORD (860) 354-5518 NORBERT E. MITCHELL CO., INC. REDDING/RIDGEFIELD (203) 431-3921 www.nemitchell.com NEW CANAAN (203) 966-5001 CT LIC. # 380200 HOD # 145 TOLL FREE (800) 237-3835 Westchester LIC. # WC-30726-H18 Basic Oil Heat Plan -- $309.95 plus tax The Basic Oil Heat Plan covers just about anything that's likely to go wrong with your burner or fuel supply system and includes an annual tune-up (see General Conditions for details) to increase your system's longevity and performance! Norbert E. Mitchell Co., Inc. will repair or replace during the life of this agreement, at no extra charge to our customer, any of the following parts which may become defective due to normal use or wear and tear. Controls Burner Replacement Parts Fuel Supply Parts Aquastat Stack Control Air Filters (one only)* Electrode Assembly Anti-hum Device Cad Cell System 2000 Manager Blower Belt End Cone Fill Cap Cad Cell Assembly Thermostat (manual) Burner Blast Tube Ignition Leads Firomatic Valve Cad Cell Relay Triple Aquastat Burner Coupling Ignition Transformer Flare Fittings Circulator Relay Burner Fan Ignition Transformer Gasket Fuel Filter Cartridge Emergency Switch Burner Flange Gasket Ignition Wiring Fuel Filter Complete Fan & Limit Control Burner Housing Low Voltage Transformer Fuel Pump Fuses (internal) Burner Motor (up to ½ HP) Nozzle Fuel Pump Bleeder High Limit Control Burner Switch Nozzle Adapter Fuel Pump Strainer Low Limit Control Buss Bar Transformer Leads Nozzle Assembly Oil Line (exposed) Pressuretrol Control Delayed Oil Valve Porcelains Oil Line Fittings (exposed) Primary Control Delayed Oil Valve Coil Solenoid Valve Oil Tank Gauge Pyrostat Electrodes 24 volt Wiring (exposed) Vent Cap Reverse Aquastat * Accordion style, Electrostatic and other specialty filters not covered. -

Application Selection and Cross-Reference Guide Support
Residential Combustion ESSENTIAL RESOURCE FOR ALL COUNTER PERSONNEL, SERVICE TECHS, AND PURCHASING AGENTS Application Selection and Cross-Reference Guide Support Online Tools Find everything you need to be successful in selling Honeywell products online. Product information, cross-references, demos and more are available at customer.honeywell.com. Customer Service Honeywell provides toll-free access to product application, installation and support experts. Technical Support: 1-800-468-1502 Customer Service: 1-888-793-8193 Training Honeywell offers a variety of on-site and web-based training options at customer.honeywell.com. Rewards Honeywell’s Contractor PRO™ Rewards program gives contractors reward points ($) back on all Honeywell purchases year round at contractorpro.com. Sales Literature With Honeywell, you’ll have access to sales materials that you can use to help grow your business. Visit literature.honeywell.com. Phone: Online: Contractor PRO: Residential Technical Support: www.customer.honeywell.com Phone: 1-800-919-4835 1-800-468-1502 www.forwardthinking.honeywell.com Fax: 1-763-954-4097 Sales Support: 1-800-328-5111 www.literature.honeywell.com Exclusive Technical Support Customer Service: 1-888-793-8193 for Contractor PRO members: Residential Zoning: 1-800-828-8367 1-877-880-3383 www.contractorpro.com 2 Table Of Contents PAGE Residential Combustion Overview 4 New Products 6 Gas Controls GAS CONTROLS Gas Valves 8 SmartValve™ System Controls 12 Ignition Modules 16 Glowfly™ Universal Hot Surface Ignitor 20 Thermocouples/Thermopiles -

Heat Transfer in Hydronic Systems
8 1 0 2 y l u j 23 JOURNAL OF DESIGN INNOVATION FOR HYDRONIC PROFESSIONALS 8 1 0 2 y l u j 23 Caleffi North America, Inc. Caleffi North America, Inc. 38839850 SouthW. Milwaukee 54th Street Rd JOURNAL OF DESIGN INNOVATION FOR HYDRONIC PROFESSIONALS Milwaukee,Franklin, WI 53132Wisconsin 53208 T:T: 414.238.2360414.421.1000 F: F: 414.421.2878 414.238.2366 Dear Plumbing and Hydronic Professional, Dear Hydronic Professional, Is an underperforming heat emitter the result of insufficient flow nd rate,Welcome low fluid to the temperature, 2 edition of idronics undersizing, – Caleffi’s improper semi-annual placement, design journal for hydronic professionals. trapped air, or sludge deposits? It could be any or all of these. The 1st edition of idronics was released in January 2007 and distributed to over 80,000 people in North America. It focused on the topic hydraulic separation. From Anthe understanding feedback received, of it’show evi dentheat we moves attained and our goalwhat of factorsexplaining enhance the benefits (orand inhibit) proper that application movement of this modernis vitally design important technique to for those hydronic who systems. A Technical Journal design, troubleshoot, or maintain hydronic systems. Conduction, If you haven’t yet received a copy of idronics #1, you can do so by sending in the from convection,attached reader and response radiation card, all or play by registering rolls, as online do material at www.caleffi.us selections. The Caleffi Hydronic Solutions andpublication the placement will be mailed of those to you materialsfree of charge. within You can a system.The also download ability the to completeestimate journal heat as transfer a PDF file rates from ourusing Web basic site.