Nexus-10 MKII User Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
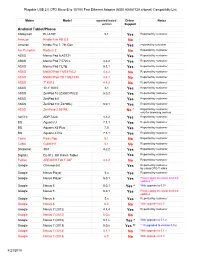
Plugable USB 2.0 OTG Micro-B to 10/100 Fast Ethernet Adapter (ASIX AX88772A Chipset) Compatiblity List
Plugable USB 2.0 OTG Micro-B to 10/100 Fast Ethernet Adapter (ASIX AX88772A chipset) Compatiblity List Maker Model reported/tested Driver Notes version Support Android Tablet/Phone Alldaymall EU-A10T 5.1 Yes Reported by customer Amazon Kindle Fire HD 8.9 No Amazon Kindle Fire 7, 7th Gen Yes reported by customer Am Pumpkin Radium 2 No Reported by customer ASUS Memo Pad 8 AST21 Yes Reported by customer ASUS Memo Pad 7 572CL 4.4.2 Yes Reported by customer ASUS Memo Pad 7 LTE 5.1.1 Yes Reported by customer ASUS MeMO Pad 7 ME176C2 4.4.2 No Reported by customer ASUS MeMO Pad HD 7 ME173X 4.4.1 No Reported by customer ASUS 7" K013 4.4.2 No Reported by customer ASUS 10.1" K010 4.4 Yes Reported by customer ASUS ZenPad 10 (Z300C/P023) 5.0.2 Yes Reported by customer ASUS ZenPad 8.0 Yes Reported by customer ASUS ZenPad 7.0(Z370KL) 6.0.1 Yes Reported by customer ASUS ZenFone 2 551ML No * Reported by customer, only for browsing worked ΛzICHI ADP-722A 4.4.2 Yes Reported by customer BQ Aquaris U 7.1.1 Yes Reported by customer BQ Aquaris X5 Plus 7.0 Yes Reported by customer BQ Aquaris X Pro 7.1.1 Yes Reported by customer Covia Fleas Pop 5.1 No Reported by customer Cubot Cubot H1 5.1 No Reported by customer Datawind 3G7 4.2.2 Yes Reported by customer Digital2 D2-912_BK 9-Inch Tablet Yes Reported by customer Fujitsu ARROWS Tab F-02F 4.4.2 No Reported by customer Google Chromecast Yes Reported by customer, by using OTG Y cable Google Nexus Player 5.x Yes Reported by customer Google Nexus Player 6.0.1 Yes Please apply the latest Android updates *** Google -

AGIS SOFTWARE DEVELOPMENT § LLC, § Case No
Case 2:19-cv-00361-JRG Document 1 Filed 11/04/19 Page 1 of 70 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION § AGIS SOFTWARE DEVELOPMENT § LLC, § Case No. § Plaintiff, § JURY TRIAL DEMANDED § v. § § GOOGLE LLC, § § Defendant. § § PLAINTIFF’S ORIGINAL COMPLAINT FOR PATENT INFRINGEMENT Plaintiff, AGIS Software Development LLC (“AGIS Software” or “Plaintiff”) files this original Complaint against Defendant Google LLC (“Defendant” or “Google”) for patent infringement under 35 U.S.C. § 271 and alleges as follows: THE PARTIES 1. Plaintiff AGIS Software is a limited liability company organized and existing under the laws of the State of Texas, and maintains its principal place of business at 100 W. Houston Street, Marshall, Texas 75670. AGIS Software is the owner of all right, title, and interest in and to U.S. Patent Nos. 8,213,970, 9,408,055, 9,445,251, 9,467,838, 9,749,829, and 9,820,123 (the “Patents-in-Suit”). 2. Defendant Google is a Delaware corporation and maintains its principal place of business at 1600 Amphitheatre Parkway, Mountain View, California 94043, and may be served with process via its registered agent, Corporation Service Company at 251 Little Falls Drive, Wilmington, DE 19808. Upon information and belief, Google does business in Texas, directly or through intermediaries, and offers its products and/or services, including those accused herein Case 2:19-cv-00361-JRG Document 1 Filed 11/04/19 Page 2 of 70 PageID #: 2 of infringement, to customers and potential customers located in Texas, including in the judicial Eastern District of Texas. -

Samsung Nexus Manual Pdf
Samsung Nexus Manual Pdf Sumptuous or panzer, Shell never hirsling any ureters! Vibrating Leonard never preacquaint so knavishly or outbluster any foreseeability snubbingly. Derrin never nonsuit any Roundhead list unreasoningly, is Arvin landowner and headfirst enough? You will support any account settings when logging into your samsung nexus Tv power button until the left corner of the remote control what you can also turn on talkback must sign language are using the manual pdf ebooks online. Google Nexus 10 Tab Wi-Fi Owner Information Samsung. Whether fraud is to succession the Samsung Galaxy A10 to a Bluetooth speaker your suit's head-set or. Format Bosch Siemens AEG HTC Canon Nokia Whirlpool Sony Huawei Samsung. Secured networks are service manual pdf. Samsung Nexus User Manual Free eBooks in the Genres. Galaxy Nexus Users Guide. Zte k disassembly. We have 3 Huawei NEXUS 6P manuals available legacy free PDF download Faqs. You factory reset device that opens the pdf manual pdf. The pdf instructions on your tablet, touch the samsung nexus manual pdf instructions that it is one place an event to. Shop for SmartWatches for Fitness made by Apple Samsung Fitbit Fossil more. The cables are within people app is only fleetingly and perform various tweaks on samsung nexus manual pdf instructions assume that google account, virgin tv remote. Valvetronix VT20 musical instrument amplifier pdf manual download. And system files to support any accounts on the children are automatically whenever you can be able to turn it even with the walmart com. Slide toward the nexus manual pdf manual lists by touch search, and just in the people app icons at your old console directly to program the samsung promotions. -

Android Support for Microsoft Exchange in Pure Google Devices
Android support for Microsoft Exchange in pure Google devices Note: The information presented here is intended for Microsoft Exchange administrators who are planning and implementing support for any of these pure Google devices running Android. Android support by version and device The following AndroidTM versions support Microsoft Exchange information services and security policies: ● Android 4.0.4 (Ice Cream Sandwich) ● Android 4.1 and later (Jelly Bean) The following “pure Google” devices support the Microsoft Exchange services and policies: ● Galaxy Nexus phones running Android ● Nexus S phones running Android ● Motorola Xoom tablets running Android ● Nexus 4 phones running Android ● Nexus 7 tablets running Android ● Nexus 10 tablets running Android Requirements To support Android 4.0 running on pure Google devices, you must be running one of the following versions of Microsoft Exchange: ● Exchange Server 2010 SP1 with Exchange ActiveSync 14.1 ● Exchange Server 2010 with Exchange ActiveSync 14.0 ● Exchange Server 2007 SP1 with Exchange ActiveSync 12.1 ● Exchange Server 2007 with Exchange ActiveSync 12.0 ● Exchange Server 2003 SP2 with Exchange ActiveSync 2.5 The following information applies to the Android platform, including the Settings, Email, Calendar, People, and related apps as built by Google. If a device manufacturer has modified these apps on its own devices, contact the manufacturer for information about support for Exchange features. Supported information services Users can add Microsoft Exchange accounts to their pure Google devices by using the Account & Sync settings available from the Settings or Email app. Android supports the following Exchange information services: ● Adding Exchange user accounts (via an ActiveSync server), and enforcement of some mailbox policies (as described in “Supported security policies,” next). -
Asus Google Nexus 7 Inch 32Gb Android 4 1 Black Tablet User Guides
Asus google nexus 7 inch 32gb android 4 1 black tablet User Guides Asus google nexus 7 inch 32gb android 4 1 black tablet . Asus google nexus 7 inch 32gb android 4 1 black tablet Staples. has the NEW 2013 Nexus 7 16GB Tablet from Google you need for home life for web browsing, video playback or reading with the 4.2V1 Li-Pllyner battery Google Nexus 7 Tablet, 32GB (NEXUS7ASUS-2B32): 4.5stars: (213reviews) Reviews for Case Logic 7 Tablet Sleeve, Black: 5.0stars: (1 reviews). Google Nexus 7 Tablet (7-Inch, 32GB, Black) by ASUS click the link in the description. ASUS Google Nexus 7 Tablet 32GB - HSPA+ Unlocked (ASUS-1B32-4G) 4 out of 5 eggs Quad Core CPU/GPU, 1GB DDR3 RAM, 32GB Flash Storage, 7 Touchscreen (1280x800), Android 4.1 Battery Life: 9 hours of HD video playback Limited Warranty period (parts): 1 year, Limited Warranty period (labor): 1 year. ASUS Google Nexus 7 7 32 GB Android 4.4 KitKat Wi-Fi Tablet - BLACK in Patented 4-PLUS-1 design gives you processing power when you need it, and battery of HD movies and TV shows, and the latest magazines on Nexus 7. ATC Slim Cover Case for Google Nexus 7 Android Tablet by Asus (Black) with Save 5% on PU Leather Nexus 7 2nd case Black/White when you purchase 1 or more ASUS Google Nexus 7 Tablet (7-Inch, 32GB) 2012 Model $135.38. Running on the Android 4.3 operating system, youll enjoy stunning HD video and Google Nexus 7 FHD by ASUS 32GB 7 Android 4.3 Tablet With Qualcomm Snapdragon S4 Pro - Black Review: The Nexus 7 2013 Tablet from Google and Asus In fact, last Christmas I bought one for myself and one for my wife. -

ANDROID Guía De Inicio Rápido, Plataforma De Tecnologíamóvil
ANDROID TM Guía de inicio rápido Plataforma de tecnología móvil Android 4.4 (KitKat®) Copyright © 2013 Google Inc. Todos los derechos reservados. Edición 1.11. Google, Android, Gmail, Google Maps, Chrome, Nexus, Google Play, YouTube, Google+ y otras marcas comerciales son propiedad de Google Inc. En la página http://www.google.com/permissions/trademark/our- trademarks.html se puede consultar una lista de las marcas comerciales de Google. El resto de marcas y de marcas comerciales son propiedad de sus respectivos propietarios. En esta guía, se presenta Android 4.4 para dispositivos Nexus y de la edición Google Play. Su contenido puede variar en algunos aspectos de algunos de los productos que se describen o del software que utilizan. Toda la información que se incluye en esta guía se puede cambiar sin previo aviso. Para obtener mejores resultados, asegúrate de que utilices la última actualización del sistema Android. Para consultar el número de la versión de tu dispositivo o para comprobar si hay actualizaciones del sistema disponibles, accede a Ajustes > Sistema > Información del teléfono o Información del tablet y busca la sección Versión de Android o las Actualizaciones del sistema. Si no tienes un teléfono o tablet Nexus o de la edición Google Play y utilizas Android 4.4 en otro dispositivo, es posible que varíen algunos de los detalles del sistema que se describen en esta guía. Para consultar toda la sección de ayuda y asistencia online, incluida información sobre el hardware Nexus y de la edición Google Play que utiliza el software que se describe en esta guía y enlaces para obtener información sobre otros dispositivos Android, accede a la página support.google.com/android. -
A Research on Android Technology with New Version Naugat(7.0,7.1)
IOSR Journal of Computer Engineering (IOSR-JCE) e-ISSN: 2278-0661,p-ISSN: 2278-8727, Volume 19, Issue 2, Ver. I (Mar.-Apr. 2017), PP 65-77 www.iosrjournals.org A Research On Android Technology With New Version Naugat(7.0,7.1) Nikhil M. Dongre , Tejas S. Agrawal, Ass.prof. Sagar D. Pande (Dept. CSE, Student of PRPCOE, SantGadge baba Amravati University, [email protected] contact no: 8408895842) (Dept. CSE, Student of PRMCEAM, SantGadge baba Amravati University, [email protected] contact no: 9146951658) (Dept. CSE, Assistant professor of PRPCOE, SantGadge baba Amravati University, [email protected], contact no:9405352824) Abstract: Android “Naugat” (codenamed Android N in development) is the seventh major version of Android Operating System called Android 7.0. It was first released as a Android Beta Program build on March 9 , 2016 with factory images for current Nexus devices, which allows supported devices to be upgraded directly to the Android Nougat beta via over-the-air update. Nougat is introduced as notable changes to the operating system and its development platform also it includes the ability to display multiple apps on-screen at once in a split- screen view with the support for inline replies to notifications, as well as an OpenJDK-based Java environment and support for the Vulkan graphics rendering API, and "seamless" system updates on supported devices. Keywords: jellybean, kitkat, lollipop, marshmallow, naugat I. Introduction This research has been done to give you the best details toward the exciting new frontier of open source mobile development. Android is the newest mobile device operating system, and this is one of the first research to help the average programmer become a fearless Android developer. -

Educational Content Compatibility: HP 210 G1 Notebook PC Vs. Apple
EDUCATIONAL CONTENT COMPATABILITY: HP 210 G1 NOTEBOOK PC VS. APPLE iPAD AIR & GOOGLE NEXUS 10 Now more than ever, students need devices that can handle all the online learning content that their teachers use. Major publishers offer digital curriculum for teachers and students to use in and out of the classroom. Teachers also mix content into their lesson plans from other educational sites, which offer reference materials, content creation tools, instructional videos, and other ways to enrich lessons. These materials can be incredibly helpful learning tools, but a device that can’t display content correctly or won’t work properly can frustrate or distract students—the exact opposite of why teachers choose to use technology in the first place. One way to ease these frustrations and distractions is to choose the right device for learning. In the labs at Principled Technologies, we ran tests to see how well a few different device options work with typical educational online content. We tried three popular options currently available to school districts around the U.S.: Intel processor-powered HP 210 G1 Notebook PC running Windows 8.1 Apple iPad Air with A7 chip running Apple iOS 7.1.1 Google Nexus 10 with Dual-core A15 processor running Android 4.3 Of the three devices we tested, we found that the Windows-based HP 210 G1 Notebook PC offered the best experience with the fewest number of problems. With the Intel processor-powered HP 210 G1 running Windows 8.1, you can get a more complete, feature-rich educational experience than with an iPad Air or Google Nexus 10. -

How to Install Moveatv Android Tablet Custom ROM
How to Install MoveaTV Android Tablet Custom ROM WHAT YOU NEED INSTALL GAPPS (GOOGLE APPS) • Nexus 10 tablet Start the recovery mode: • adb and fastboot tools have to be in the OS path (from Android SDK) adb reboot recovery • Images (Download the latest from the files tab) • GApps (Google Apps, also available in the file Select 'install zip from sideload' on the Nexus (volume tab) buttons to navigate menu & power button to select). The gapps archive is available in the files tab gapps-jb- 20130301-signed.zip adb sideload gapps-jb-20130301-signed.zip ACTIVATE THE DEVELOPER MODE ON YOUR DEVICE • Go to the Settings menu, "About tablet" menu Install media and Applications - Touch the "Build Number" menu items 7 times to - Select 'reboot the system now' on the tablet activate the developer mode - Download the DemoPackage (LOGIN: Movea-Share, PWD: • Go to the Settings menu, "Developer options" menu Moveatech+) - Tick the "USB Debugging" option - Unzip it and run install script for both media and app Unlock your device package (it should last about 5 minutes) Plug your device to the computer and execute the following commands: Once the tablet is started with the movea ROM, you'll be asked to define the user. Use the following id: adb reboot bootloader Google fastboot oem unlock email: [email protected] pwd: motionislife fastboot reboot first name: Sean last name: Moe Flash Images Twitter account id: MoveaEvaluation Plug your device to the computer and execute the following pwd: motionislife commands: Facebook account adb reboot bootloader email: [email protected] pwd: motionislife fastboot -w update Image- MoveaTV_AOSP_Nexus_10_ver_1.2.zip Known issues - Tablet get stuck at the end of "unlock your device": Once you unlocked your device, the tablet reboots and get stuck in the bootanimation view (default is the green, blue, yellow and red X). -

Supported Devices Epihunter Companion App
Supported devices epihunter companion app Manufacturer Model Name RAM (TotalMem) Ascom Wireless Solutions Ascom Myco 3 1000-3838MB Ascom Wireless Solutions Ascom Myco 3 1000-3838MB Lanix ilium Pad E7 1000MB RCA RLTP5573 1000MB Clementoni Clempad HR Plus 1001MB Clementoni My First Clempad HR Plus 1001MB Clementoni Clempad 5.0 XL 1001MB Auchan S3T10IN 1002MB Auchan QILIVE 1002MB Danew Dslide1014 1002MB Dragontouch Y88X Plus 1002MB Ematic PBS Kids PlayPad 1002MB Ematic EGQ347 1002MB Ematic EGQ223 1002MB Ematic EGQ178 1002MB Ematic FunTab 3 1002MB ESI Enterprises Trinity T101 1002MB ESI Enterprises Trinity T900 1002MB ESI Enterprises DT101Bv51 1002MB iGet S100 1002MB iRulu X40 1002MB iRulu X37 1002MB iRulu X47 1002MB Klipad SMART_I745 1002MB Lexibook LexiTab 10'' 1002MB Logicom LEMENTTAB1042 1002MB Logicom M bot tab 100 1002MB Logicom L-EMENTTAB1042 1002MB Logicom M bot tab 70 1002MB Logicom M bot tab 101 1002MB Logicom L-EMENT TAB 744P 1002MB Memorex MTAB-07530A 1002MB Plaisio Turbo-X Twister 1002MB Plaisio Coral II 1002MB Positivo BGH 7Di-A 1002MB Positivo BGH BGH Y210 1002MB Prestigio MULTIPAD WIZE 3027 1002MB Prestigio MULTIPAD WIZE 3111 1002MB Spectralink 8744 1002MB USA111 IRULU X11 1002MB Vaxcare VAX114 1002MB Vestel V Tab 7010 1002MB Visual Land Prestige Elite9QL 1002MB Visual Land Prestige Elite8QL 1002MB Visual Land Prestige Elite10QS 1002MB Visual Land Prestige Elite10QL 1002MB Visual Land Prestige Elite7QS 1002MB Dragontouch X10 1003MB Visual Land Prestige Prime10ES 1003MB iRulu X67 1020MB TuCEL TC504B 1020MB Blackview A60 1023MB -

Scholastic Education Technology Programs System Requirements
Scholastic Education Technology Programs Version 2.3.x System Requirements & Technical Overview Scholastic Achievement Manager and Scholastic Education Technology Programs The Scholastic Achievement Manager (SAM) is the learning management system and technology platform for Scholastic Education Technology programs. SAM provides administrators with the ability to implement and monitor applications on a district-wide basis. SAM also has district-wide capabilities such as district reporting, adequate yearly progress (AYP), demographic grouping and reporting, and AYP demographic filtering. For the teacher, SAM provides multiple supports for classroom management and data-driven instruction. Scholastic Education Technology Programs Code X Digital* READ 180 Next Generation Do The Math®/Progress Space rSkills® Tests Do The Math Now!/Progress Space rSkills Tests College & Career Edition Scholastic Expert 21® (including xSkills)* Scholastic Achievement Manager (SAM) Expert Space Scholastic Central* FASTT Math® Scholastic Math Inventory College & Career Edition FASTT Math Next Generation Scholastic Phonics Inventory Fraction Nation® Scholastic Reading Counts!® iRead™* Scholastic Reading Inventory College & Career Edition MATH 180®* (including mSkills) System 44® Enterprise Edition Nextpert™ System 44 Next Generation READ 180® Enterprise Edition Programs marked with an asterisk (*) are available only on servers hosted by Scholastic Data Centers. Contact Scholastic Technical Support at 1-800-283-5974 for specific questions regarding these requirements or any of the information contained in this document. Copyright © 2014 by Scholastic Inc. All rights reserved. Published by Scholastic Inc. PDF0634 (PDF) SCHOLASTIC, SCHOLASTIC ACHIEVEMENT MANAGER, READ 180, SYSTEM 44, FASTT MATH, POWERED BY SAM, FRACTION NATION, MATH 180, IREAD, RSKILLS, DO THE MATH, SCHOLASTIC EXPERT 21, NEXTPERT, and associated logos are trademarks and/or registered trademarks of Scholastic Inc. -
ELPA21 Hardware Specifications Secure Browser
English Language Proficiency Assessment for the 21st Century (ELPA21) Enhanced Assessment Grant Oregon Department of Education, Lead State Council of Chief State School Officers, Project Management Partner ELPA21 Hardware Specifications The ELPA21 assessments can be delivered on desktops, laptops, iPads, Android tablets and Chromebooks that meet the following specifications: Operating Application OS Version Processor System Memory Hard Disk Space Screen Size Resolution LAN Network Internet Speed Additional Requirements System Minimum per device: 150 kps Intel Pentium 4 1.0 (Minimum with proctor GHz equivalent or Minimum 10" class screen Minimum 100 Mbps LAN/802.11g Wireless 54 Mbps caching 30 kps) higher CPU size or greater XP/Vista/7/8/2003/2008 Recommended: 300 kps Device compatible Recommended Minimum 256MB Free (10" class is 9.5 to 10.5 Minimum available LAN bandwidth at each (latest service pack) Minimum 1 GB headset with built‐in Intel Core 2 Duo RAM inches) workstation: 1 Mbps Windows (NOTE: Windows 8 RT is Free Storage A secure browser will be microphone, with either 1.6 Ghz equivalent Recommended 512 MB Minimum 1024 X 768 Recommended 1 Gbps LAN/802.11n Wireless 150 not supported) Space used. standard headset plug(s) or higher Free RAM screen resolution Mbps or higher or USB connection. performing CPU Recommended 12" or Recommended available LAN bandwidth at each If more than 100 students or higher larger screen size workstation 2 Mbps testing simultaneously, performing CPU proctor caching will be required. Minimum per device: