Behavioral, Morphological, and Ecological Trait Evolution in Two Clades of New World Sparrows (Aimophila and Peucaea, Passerellidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comprehensive Multilocus Assessment of Sparrow (Aves: Passerellidae) Relationships ⇑ John Klicka A, , F
Molecular Phylogenetics and Evolution 77 (2014) 177–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication A comprehensive multilocus assessment of sparrow (Aves: Passerellidae) relationships ⇑ John Klicka a, , F. Keith Barker b,c, Kevin J. Burns d, Scott M. Lanyon b, Irby J. Lovette e, Jaime A. Chaves f,g, Robert W. Bryson Jr. a a Department of Biology and Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA 98195-3010, USA b Department of Ecology, Evolution, and Behavior, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA c Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA d Department of Biology, San Diego State University, San Diego, CA 92182, USA e Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA f Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA g Universidad San Francisco de Quito, USFQ, Colegio de Ciencias Biológicas y Ambientales, y Extensión Galápagos, Campus Cumbayá, Casilla Postal 17-1200-841, Quito, Ecuador article info abstract Article history: The New World sparrows (Emberizidae) are among the best known of songbird groups and have long- Received 6 November 2013 been recognized as one of the prominent components of the New World nine-primaried oscine assem- Revised 16 April 2014 blage. Despite receiving much attention from taxonomists over the years, and only recently using molec- Accepted 21 April 2014 ular methods, was a ‘‘core’’ sparrow clade established allowing the reconstruction of a phylogenetic Available online 30 April 2014 hypothesis that includes the full sampling of sparrow species diversity. -

Artificial Water Catchments Influence Wildlife Distribution in the Mojave
The Journal of Wildlife Management; DOI: 10.1002/jwmg.21654 Research Article Artificial Water Catchments Influence Wildlife Distribution in the Mojave Desert LINDSEY N. RICH,1,2 Department of Environmental Science, Policy, and Management, University of California- Berkeley, 130 Mulford Hall 3114, Berkeley, CA 94720, USA STEVEN R. BEISSINGER, Department of Environmental Science, Policy, and Management, University of California- Berkeley, 130 Mulford Hall 3114, Berkeley, CA 94720, USA JUSTIN S. BRASHARES, Department of Environmental Science, Policy, and Management, University of California- Berkeley, 130 Mulford Hall 3114, Berkeley, CA 94720, USA BRETT J. FURNAS, Wildlife Investigations Laboratory, California Department of Fish and Wildlife, Rancho Cordova, CA 95670, USA ABSTRACT Water often limits the distribution and productivity of wildlife in arid environments. Consequently, resource managers have constructed artificial water catchments (AWCs) in deserts of the southwestern United States, assuming that additional free water benefits wildlife. We tested this assumption by using data from acoustic and camera trap surveys to determine whether AWCs influenced the distributions of terrestrial mammals (>0.5 kg), birds, and bats in the Mojave Desert, California, USA. We sampled 200 sites in 2016–2017 using camera traps and acoustic recording units, 52 of which had AWCs. We identified detections to the species-level, and modeled occupancy for each of the 44 species of wildlife photographed or recorded. Artificial water catchments explained spatial variation in occupancy for 8 terrestrial mammals, 4 bats, and 18 bird species. Occupancy of 18 species was strongly and positively associated with AWCs, whereas 1 species (i.e., horned lark [Eremophila alpestris]) was negatively associated. Access to an AWC had a larger influence on species’ distributions than precipitation and slope and was nearly as influential as temperature. -

A COMPARATIVE ANALYSIS of SONG and RESPONSES to SONG PLAYBACK University of Colorado at Denver
FINAL REPORT A COMPARATIVE ANALYSIS OF SONG AND RESPONSES TO SONG PLAYBACK IN THE AVIAN GENUS PIPILO Peter S. Kaplan Department of Psychology University of Colorado at Denver Abstract An experiment was undertaken to characterize the responses of Green-tailed Towhees (Pipilo chlorura) and Rufous-sided Towhees (P. erythophthalmus) to each others' songs and to the songs of five other towhee species, plus one hybrid form. A total of 12 Green-Tailed Towhees and 10 Rufous-sided Towhees from Boulder and Gilpin counties were studied at three field sites: the Doudy Draw Trail, the National Center for Atmospheric Research (NCAR), and on private land at the mouth of Coal Creek Canyon. In May, each bird was mist-netted and banded to facilitate individual identification. During the subsequent playback phase in June and July, each individual received one three-part playback trial on each of 7 consecutive or near-consecutive days. A 9-min playback trial consisted of a 3-min "pre-play" period, during which the bird was observed in the absence of song playback, a 3-min "play" period, in which tape recorded song was played to the subject from a central point in his territory, and a 3-min "post-play" period when the bird • was again observed in the absence of song playback. Order of presentation of song exemplars from different towhee species were randomized across birds. The main dependent measure was the change in the number of songs produced by the subject bird during song playback, relative to the pre-play period. Results showed that Green-tailed Towhees responded by significantly increasing their rate of singing, but only in response to Green-tailed Towhee songs. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

Life History Account for California Towhee
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group CALIFORNIA TOWHEE Melozone crissalis Family: EMBERIZIDAE Order: PASSERIFORMES Class: AVES B484 Written by: D. Dobkin, S. Granholm Reviewed by: L. Mewaldt Edited by: R. Duke Updated by: CWHR Program Staff, November 2014 DISTRIBUTION, ABUNDANCE, AND SEASONALITY The former brown towhee recently has been split into the California towhee and the canyon towhee, M. fusca (American Ornithologists' Union 1989). The California towhee is a common, characteristic resident of foothills and lowlands in most of cismontane California. Frequents open chaparral and coastal scrub, as well as brush-land patches in open riparian, hardwood hardwood-conifer, cropland, and urban habitats. Commonly uses edges of dense chaparral and brushy edges of densely wooded habitats. Also occurs in lowest montane habitats of similar structure in southern California, and locally in Siskiyou and western Modoc cos. Local on coastal slope north of southern Humboldt Co., and apparently absent from western San Joaquin Valley (Grinnell and Miller 1944, McCaskie et al. 1979, Garrett and Dunn 1981). The Inyo California towhee, M. c. eremophilus, occurs only in the Argus Mountains of southwestern Inyo Co. SPECIFlC HABITAT REQUIREMENTS Feeding: Feeds on seeds, insects, and some fruits. Gleans and scratches in litter, picks seeds and fruits from plants, and rarely flycatches (Davis 1957). Prefers to forage on open ground adjacent to brushy cover. Insects are important in breeding season, often constituting a third of the diet (Martin et al. 1961). Cover: Shrubs in broken chaparral, margins of dense chaparral, willow thickets, and brushy understory of open wooded habitats provide cover. -

Wildland Fire in Ecosystems: Effects of Fire on Fauna
United States Department of Agriculture Wildland Fire in Forest Service Rocky Mountain Ecosystems Research Station General Technical Report RMRS-GTR-42- volume 1 Effects of Fire on Fauna January 2000 Abstract _____________________________________ Smith, Jane Kapler, ed. 2000. Wildland fire in ecosystems: effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 83 p. Fires affect animals mainly through effects on their habitat. Fires often cause short-term increases in wildlife foods that contribute to increases in populations of some animals. These increases are moderated by the animals’ ability to thrive in the altered, often simplified, structure of the postfire environment. The extent of fire effects on animal communities generally depends on the extent of change in habitat structure and species composition caused by fire. Stand-replacement fires usually cause greater changes in the faunal communities of forests than in those of grasslands. Within forests, stand- replacement fires usually alter the animal community more dramatically than understory fires. Animal species are adapted to survive the pattern of fire frequency, season, size, severity, and uniformity that characterized their habitat in presettlement times. When fire frequency increases or decreases substantially or fire severity changes from presettlement patterns, habitat for many animal species declines. Keywords: fire effects, fire management, fire regime, habitat, succession, wildlife The volumes in “The Rainbow Series” will be published during the year 2000. To order, check the box or boxes below, fill in the address form, and send to the mailing address listed below. -

Voice in Communication and Relationships Among Brown Towhees
THE CONDOR VOLUME 66 SEPTEMBER-OCTOBER, 1964 NUMBER 5 VOICE IN COMMUNICATION AND RELATIONSHIPS AMONG BROWN TOWHEES By JOET.MARSHALL,JR. This paper seeks to answer two questions: (1) What is the function of each song and call in brown towhees; that is, what information does a bird communicate to its fellows vocally, o’r how does it regulate their behavior by its voice? (2) What evi- dence does voice offer for understanding relationship by descent within the closely-knit group of brown towhee species? For the first, I would extend the analysis of Quain- tance (1938,194l) to all members of the group. As to the second question, an ingenious evolutionary reconstruction, based on museum and habitat studies, has been developed by Davis (1951). Do vocal attributes agree with his scheme? The three speciesof brown towhees, genus Pipdo, are the same size and general color and are more similar to each other than any one of them is to other ground-inhabiting finches in the same genus and in the genus Melozorte. Indeed, so close is their relation- ship that the same calls can easily be discerned in each species; although differing in timbre, similarity in form and usage proclaims them to be homologous. The Abert Towhee (Pipdo abed) occupies dense riparian woodland and mesquite thickets of the Colorado River and Gila River drainages, mostly in Arizona. The Brown Towhee proper (Pip20 fuscus) lives in brushy margins of openings in the southwestern United States and Mexico. The White-throated Towhee (Pipilo aZbicoZZis)inhabits brushy slopes, often with tree yuccas, in Puebla and Oaxaca, MCxico. -
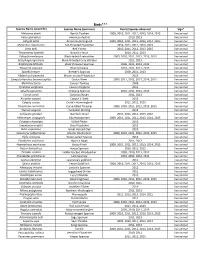
Flora and Fauna List.Xlsx
Birds*** Species Name (scientific) Species Name (common) Year(s) Species observed Sign* Melozone aberti Abert's Towhee 2009, 2010, 2011, 2012, 2013, 2014, 2015 live animal Falco sparverius American Kestrel 2010, 2013 live animal Calypte anna Anna's Hummingbird 2009, 2010, 2011, 2012, 2013, 2014, 2015 live animal Myiarchus cinerascens Ash‐throated Flycatcher 2010, 2011, 2012, 2013, 2015 live animal Vireo bellii Bell's Vireo 2010, 2011, 2012, 2013, 2015 live animal Thryomanes bewickii Bewick's Wren 2010, 2011, 2013 live animal Polioptila melanura Black‐tailed Gnatcatcher 2009, 2010, 2011, 2012, 2013, 2015 live animal Setophaga nigrescens Black‐throated Gray Warbler 2011, 2013 live animal Amphispiza bilineata Black‐throated Sparrow 2009, 2011, 2012, 2013 live animal Passerina caerulea Blue Grosbeak 2010, 2011, 2012, 2013 live animal Spizella breweri Brewer's Sparrow 2009, 2011, 2013 live animal Myiarchus tryannulus Brown‐crested Flycatcher 2013 live animal Campylorhynchus brunneicapillus Cactus Wren 2009, 2011, 2012, 2013, 2014, 2015 live animal Melozone fusca Canyon Towhee 2009 live animal Tyrannus vociferans Cassin's Kingbird 2011 live animal Spizella passerina Chipping Sparrow 2010, 2011, 2012, 2013 live animal Corvus corax Common Raven 2011, 2013 live animal Accipiter cooperii Cooper's Hawk 2013 live animal Calypte costae Costa's Hummingbird 2011, 2012, 2013 live animal Toxostoma curvirostre Curve‐billed Thrasher 2009, 2010, 2011, 2012, 2013, 2015 live animal Sturnus vulgaris European Starling 2012 live animal Callipepla gambelii Gambel's -
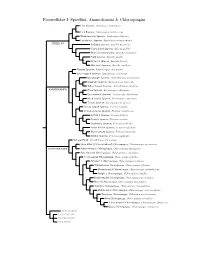
Passerellidae Species Tree
Passerellidae I: Spizellini, Ammodramini & Chlorospingini Lark Sparrow, Chondestes grammacus Lark Bunting, Calamospiza melanocorys Black-throated Sparrow, Amphispiza bilineata Five-striped Sparrow, Amphispiza quinquestriata SPIZELLINI Chipping Sparrow, Spizella passerina Clay-colored Sparrow, Spizella pallida Black-chinned Sparrow, Spizella atrogularis Field Sparrow, Spizella pusilla Brewer’s Sparrow, Spizella breweri Worthen’s Sparrow, Spizella wortheni Tumbes Sparrow, Rhynchospiza stolzmanni Stripe-capped Sparrow, Rhynchospiza strigiceps Grasshopper Sparrow, Ammodramus savannarum Grassland Sparrow, Ammodramus humeralis Yellow-browed Sparrow, Ammodramus aurifrons AMMODRAMINI Olive Sparrow, Arremonops rufivirgatus Green-backed Sparrow, Arremonops chloronotus Black-striped Sparrow, Arremonops conirostris Tocuyo Sparrow, Arremonops tocuyensis Rufous-winged Sparrow, Peucaea carpalis Cinnamon-tailed Sparrow, Peucaea sumichrasti Botteri’s Sparrow, Peucaea botterii Cassin’s Sparrow, Peucaea cassinii Bachman’s Sparrow, Peucaea aestivalis Stripe-headed Sparrow, Peucaea ruficauda Black-chested Sparrow, Peucaea humeralis Bridled Sparrow, Peucaea mystacalis Tanager Finch, Oreothraupis arremonops Short-billed (Yellow-whiskered) Chlorospingus, Chlorospingus parvirostris CHLOROSPINGINI Yellow-throated Chlorospingus, Chlorospingus flavigularis Ashy-throated Chlorospingus, Chlorospingus canigularis Sooty-capped Chlorospingus, Chlorospingus pileatus Wetmore’s Chlorospingus, Chlorospingus wetmorei White-fronted Chlorospingus, Chlorospingus albifrons Brown-headed -

NEWS and NOTES by Paul Hess
NEWS AND NOTES by Paul Hess “Lilian’s” Meadowlark für Ornithologie 135:28), but no details about the findings have been published. Ornithologist Harry C. Oberholser was intrigued by mead - At last, a large-scale genetic report by F. Keith Barker, Ar - owlarks collected in 1929 near Arizona’s Huachuca Moun - ion J. Vandergon, and Scott M. Lanyon arrived in 2008 tains, because their size, structure, and plumage differed (Auk 125:869–879). They examined two mitochondrial from those of the Western Meadowlark and from known DNA (mtDNA) genes and one nuclear locus in 14 Eastern variations of Eastern Meadowlark. In 1930 he described the Meadowlark subspecies throughout the North American, specimens as a new Eastern Meadowlark subspecies and Central American, and South American range. named it lilianae to honor Lilian Baldwin, who had donat - The results indicate a long history of evolutionary diver - ed the collection ( Scientific Publications of the Cleveland Mu - gence between lilianae and all except one other Eastern seum of Natural History 1:83–124). Meadowlark subspecies, auropectoralis of central and We now know it as “Lilian’s” Meadowlark, of desert grasslands in the southwestern U.S. and northern Mexico, where it is distinguishable from Western Meadowlark with careful study by eye and ear. In identification guides, lilianae re - ceived little attention and no illustration until the National Geographic Field Guide to the Birds of North America in 1983. Kevin J. Zimmer of - fered tips in Birding (August 1984, pp. 155–156) and in his books The Western Bird Watcher (Prentice-Hall 1985) and Birding in the American West (Cornell University Press 2000). -
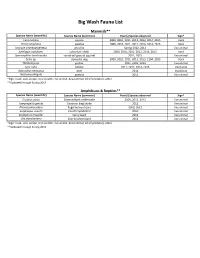
Flora and Fauna List.Xlsx
Big Wash Fauna List Mammals** Species Name (scientific) Species Name (common) Year(s) Species observed Sign* Canis latrans coyote 2009, 2010, 2011, 2012, 2013, 2014, 2015 track Pecari angulatus javalina 2009, 2010, 2011, 2012, 2013, 2014, 2015 track Urocyon cinereoargenteus gray fox Spring 2012, 2013 live animal Sylvilagus audubonii cottontail rabbit 2009, 2010, 2011, 2012, 2013, 2015 track Spermophilus tereticaudus round‐tail ground squirrel 2011, 2015 live animal Canis sp. domestic dog 2009, 2010, 2011, 2012, 2013, 2104, 2015 track Thomomys sp. gopher 2011, 2102, 2013 live animal Lynx rufus bobcat 2011, 2012, 2014, 2015 track/scat Odocoileus hemionus deer 2011 track/scat Neotoma albigula packrat 2011 live animal *Sign: track, scat, scrape, tree scratch, live animal, dead animal, kill (if predator), other **Updated through Spring 2015 Amphibians & Reptiles** Species Name (scientific) Species Name (common) Year(s) Species observed Sign* Crotalus atrox Diamondback rattlesnake 2009, 2012, 2013 live animal Lampropeltis getula Common king Snake 2012 live animal Phrynosoma solare Regal horned lizard 2009, 2012 live animal Scaphiopus couchii Couch's Spadefoot 2010 live animal Sceloporus magister Spiny lizard 2013 live animal Uta stansburiana Side‐bloched lizard 2013 live animal *Sign: track, scat, scrape, tree scratch, live animal, dead animal, kill (if predator), other **Updated through Spring 2015 Birds*** Species Name (scientific) Species Name (common) Year(s) Species observed Sign* Melozone aberti Abert's Towhee 2009, 2010, 2011, 2012, -

Program for 70Th Annual Meeting of the COOPER ORNITHOLOGICAL SOCIETY Riverside, California, 25 - 29 April, 2000
Program and Abstracts Cooper Ornithological Society 70th Annual Meeting 25-29 April 2000 Riverside, California Program for 70th Annual Meeting of the COOPER ORNITHOLOGICAL SOCIETY Riverside, California, 25 - 29 April, 2000 Local Hosts University of California, Riverside Campus University of California Natural Reserve System University of California Cooperative Extension Committee on Local Arrangements John Rotenberry, Chair Tom Scott, Co-chair Paul Aigner Cathy Koehler Karen Bagne Bill Kristan Jutta Burger Michael Patten Sharon Coe Kris Preston Jill Deppe Tim Redman Ian Gillespie Bill Webb Sheila Kee Walter Wehtje Scientific Program Committee Tom Scott, Chair John Rotenberry, Co-chair Charles Collins Kimball Garrett Hugh Ellis Mark Reynolds Steve Zack Sponsors US Geological Survey US Fish and Wildlife Service Region 6 UC Riverside College of Natural and Agricultural Sciences San Bernardino Valley Audubon Society UC Riverside Natural Reserve System UC Cooperative Extension Integrated Hardwood Range Management Program Lynx Edicions Oxford University Press University of Alaska Press Thayer Birding Software UNIVERSITY Habitat Characteristics William D . Berry OF ALASKA of Some Passerine Birds 1954-1956 Alaskan Field Sketches PRESS in Western North American Taiga compiled by Elizabeth Berry • Brina Kessel 1989, paper, reg . $19.95 conf. special $12.00 1998, paper, reg . $16.95 conf, special $13 cloth, reg .95 conf. special $25 To contact us .50 . $39 .00 Over one-third of these eloquent and detailed visit our website: This book describes specific habitat relation- sketches capture a range of Alaska birds . h ttp ://WWW. uaf.ed u/ ships of some of the small land birds that live in the subarctic . It contains descriptions and Accompanied by detailed field notes, the images uapress provide an intimate view of their activities and photographs of typical taiga habitats, color plates of the major bird species discussed, and habitats from hatchlings to adults .