Species Relationships in the Avian Genus Aimophila
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6.5 Coastal Cactus Wren (Campylorhynchus Brunneicapillus Sandiegensis) – Category SO Management Units with Known Occurrences
Volume 2D: Goals and Objectives for Species Focus Management Species 6.0 Birds 6.5 Coastal Cactus Wren (Campylorhynchus brunneicapillus sandiegensis) – Category SO Management Units with Known Occurrences Coastal cactus wrens are restricted to cactus-dominated coastal sage scrub habitats in Southern California, from Ventura south to San Diego County and inland to western San Bernardino and western Riverside Counties. These wrens differ ecologically from more common desert wrens in the southwestern United States and northern Mexico. Coastal cactus wrens began significantly declining in San Diego County in the early 1980s due to habitat loss to agriculture and urban development (Rea and Weaver 1990). By 1990 there was a 33% population decline from the previous decade as a result of the loss of coastal birds and smaller populations, and a decline in abundance of remaining populations. Coastal cactus wren surveys and cactus mapping were implemented on Conserved Lands in the MSPA in 2009 and 2011 (USFWS 2011). Cactus wrens were documented on Conserved Lands in MUs 1, 2, 3, 4, 5, and 6 (see Occurrence Table and online map: http://arcg.is/2kU1bka). A range-wide genetics and banding study was conducted across occupied cactus scrub habitats in 2011–2013 by USGS to determine coastal cactus wren population genetic structure, connectivity, and genetic diversity in Southern California (Barr et al. 2015). The study found 3 main genetic clusters in San Diego County: Otay; San Diego/El Cajon (Sweetwater/Encanto/Lake Jennings); and San Pasqual. In the San Diego/El Cajon genetic cluster, wrens in the Sweetwater River watershed are connected to occurrences in Fletcher Hills and Lake Jennings to the northeast in MU4 and to occurrences in Encanto Canyon and other urban canyons to the west in MU2. -

Assessing Bird Species Richness Within Shade-Grown Coffee Farms in Chiapas, Mexico / Project ID: 0251711
Assessing Bird Species Richness within Shade-Grown Coffee Farms in Chiapas, Mexico / Project ID: 0251711 Daniel Camilo Thompson Poo, Daniela Valle León, Alberto Martínez Fernández and Jennifer Siobhan Lowry San Cristóbal de las Casas, Chiapas, México. C.P. 29200 / [email protected] 10 July, 2012. Revised December 2014 Assessing Bird Species Richness within Shade-Grown Coffee Farms in Chiapas, Mexico / ID: 0251711 Overall Aim The goal of this project was to identify mechanisms and conservation strategies across agro-forestry systems in the El Triunfo Biosphere Reserve in Chiapas, Mexico. In particular we analyzed key biodiversity, economic, and social components that impact land-use change and ecosystem services in coffee production areas, focusing on how to improve sustainable production and conservation of nature. 2 Assessing Bird Species Richness within Shade-Grown Coffee Farms in Chiapas, Mexico / ID: 0251711 Section 1 Summary The agroforestry systems with coffee at the Sierra Madre of Chiapas, as a part of the Mesoamerican Biological Corridor region, are important for bird species. Agroforestry ecosystems also represent sustainable livelihoods for indigenous groups on the region. Sustainable coffee farming system represents a less human impact on the ecosystem. However, not all coffee producers on the region produce on the same way. Not all the inhabitants are aware of the importance of birds, as a part of the great natural capital of la Sierra Madre, but they either are prepared for the climate change risks and impacts. In this sense, this project seeks to understand, generate and communicate information useful for coffee farmers and their families. The goal is to understand social and economic factors to maintain and increase agroforestry systems with sustainable coffee. -

Campylorhynchus Rufinucha) Rufinucha) (Campylorhynchus
Nest-site characteristics of Rufous-naped Wrens Artículo (Campylorhynchus rufinucha) in Acacia trees may serve to avoid vertebrate predators Características de sitios de los nidos de Campylorhynchus rufinucha en árboles de Acacia posiblemente sirven para evadir depredación por vertebrados Ignacio Escalante1 1Sistema de Estudios de Posgrado, Escuela de Biología, Universidad de Costa Rica. San José, Costa Rica. Ornitología Colombiana Ornitología [email protected] Abstract The high rate of nest predation in tropical birds results in strong selection pressure. The Rufous-naped Wren (Campylorhynchus rufinucha) nests in Bullhorn trees (Acacia) in the Mesoamerican dry forest. It has been proposed that bullhorns and their aggressive ants (Pseudomyrmex spp.) help to prevent nest predation. I tested the hypothesis that these birds place their nests in particular acacia micro- habitats to avoid predation by vertebrates such as tufted capuchin monkeys (Cebus capucinus). I expected to find nests in locations that avoided the foraging behavior preferences of the monkeys. Along 6 km gravel road in Palo Verde National Park, Guanacaste, Costa Rica, I found 52 Rufous-naped Wren nests. The proportion of ant species in acacias with nests did not differ from the background proportion of ant species in acacias without nests, so birds did not prefer to nest in acacias with the most aggressive ant species. Acacia trees with wren nests were larger in diameter than control acacias without nests. I found more nests in acacias that were clustered, which consisted of one to five acacias in a 3m radius plot around the acacia with nest. However, the number of acacias with or without nests did not differ in their isolation from other non-acacia trees. -

A Comprehensive Multilocus Assessment of Sparrow (Aves: Passerellidae) Relationships ⇑ John Klicka A, , F
Molecular Phylogenetics and Evolution 77 (2014) 177–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication A comprehensive multilocus assessment of sparrow (Aves: Passerellidae) relationships ⇑ John Klicka a, , F. Keith Barker b,c, Kevin J. Burns d, Scott M. Lanyon b, Irby J. Lovette e, Jaime A. Chaves f,g, Robert W. Bryson Jr. a a Department of Biology and Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA 98195-3010, USA b Department of Ecology, Evolution, and Behavior, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA c Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA d Department of Biology, San Diego State University, San Diego, CA 92182, USA e Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA f Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA g Universidad San Francisco de Quito, USFQ, Colegio de Ciencias Biológicas y Ambientales, y Extensión Galápagos, Campus Cumbayá, Casilla Postal 17-1200-841, Quito, Ecuador article info abstract Article history: The New World sparrows (Emberizidae) are among the best known of songbird groups and have long- Received 6 November 2013 been recognized as one of the prominent components of the New World nine-primaried oscine assem- Revised 16 April 2014 blage. Despite receiving much attention from taxonomists over the years, and only recently using molec- Accepted 21 April 2014 ular methods, was a ‘‘core’’ sparrow clade established allowing the reconstruction of a phylogenetic Available online 30 April 2014 hypothesis that includes the full sampling of sparrow species diversity. -

Predation on Vertebrates by Neotropical Passerine Birds Leonardo E
Lundiana 6(1):57-66, 2005 © 2005 Instituto de Ciências Biológicas - UFMG ISSN 1676-6180 Predation on vertebrates by Neotropical passerine birds Leonardo E. Lopes1,2, Alexandre M. Fernandes1,3 & Miguel Â. Marini1,4 1 Depto. de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, 31270-910, Belo Horizonte, MG, Brazil. 2 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, Pampulha, 31270-910, Belo Horizonte, MG, Brazil. E-mail: [email protected]. 3 Current address: Coleções Zoológicas, Aves, Instituto Nacional de Pesquisas da Amazônia, Avenida André Araújo, 2936, INPA II, 69083-000, Manaus, AM, Brazil. E-mail: [email protected]. 4 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Biologia, Universidade de Brasília, 70910-900, Brasília, DF, Brazil. E-mail: [email protected] Abstract We investigated if passerine birds act as important predators of small vertebrates within the Neotropics. We surveyed published studies on bird diets, and information on labels of museum specimens, compiling data on the contents of 5,221 stomachs. Eighteen samples (0.3%) presented evidence of predation on vertebrates. Our bibliographic survey also provided records of 203 passerine species preying upon vertebrates, mainly frogs and lizards. Our data suggest that vertebrate predation by passerines is relatively uncommon in the Neotropics and not characteristic of any family. On the other hand, although rare, the ability to prey on vertebrates seems to be widely distributed among Neotropical passerines, which may respond opportunistically to the stimulus of a potential food item. -

Artificial Water Catchments Influence Wildlife Distribution in the Mojave
The Journal of Wildlife Management; DOI: 10.1002/jwmg.21654 Research Article Artificial Water Catchments Influence Wildlife Distribution in the Mojave Desert LINDSEY N. RICH,1,2 Department of Environmental Science, Policy, and Management, University of California- Berkeley, 130 Mulford Hall 3114, Berkeley, CA 94720, USA STEVEN R. BEISSINGER, Department of Environmental Science, Policy, and Management, University of California- Berkeley, 130 Mulford Hall 3114, Berkeley, CA 94720, USA JUSTIN S. BRASHARES, Department of Environmental Science, Policy, and Management, University of California- Berkeley, 130 Mulford Hall 3114, Berkeley, CA 94720, USA BRETT J. FURNAS, Wildlife Investigations Laboratory, California Department of Fish and Wildlife, Rancho Cordova, CA 95670, USA ABSTRACT Water often limits the distribution and productivity of wildlife in arid environments. Consequently, resource managers have constructed artificial water catchments (AWCs) in deserts of the southwestern United States, assuming that additional free water benefits wildlife. We tested this assumption by using data from acoustic and camera trap surveys to determine whether AWCs influenced the distributions of terrestrial mammals (>0.5 kg), birds, and bats in the Mojave Desert, California, USA. We sampled 200 sites in 2016–2017 using camera traps and acoustic recording units, 52 of which had AWCs. We identified detections to the species-level, and modeled occupancy for each of the 44 species of wildlife photographed or recorded. Artificial water catchments explained spatial variation in occupancy for 8 terrestrial mammals, 4 bats, and 18 bird species. Occupancy of 18 species was strongly and positively associated with AWCs, whereas 1 species (i.e., horned lark [Eremophila alpestris]) was negatively associated. Access to an AWC had a larger influence on species’ distributions than precipitation and slope and was nearly as influential as temperature. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

Acacia, Cattle and Migratory Birds in Southeastern Mexico
Biological Conservation 80 (1997) 235-247 © 1997 Elsevier Science Ltd All rights reserved. Printed in Great Britain PII: S0006-3207(96)00137- 1 0006-3207/97 $17.00 + 0.00 ELSEVIER ACACIA, CATTLE AND MIGRATORY BIRDS IN SOUTHEASTERN MEXICO Russell Greenberg,* Peter Bichier & John Sterling Smithsonian Migratory Bird Center, National Zoological Park, Washington, DC 20008, USA (Accepted 11 July 1996) Abstract of forest-dependent migratory birds (Askins et al., Acacia pennatula groves in mid-elevation valleys of 1990). However, the new tropical landscape is usually a southern Mexico supported both the highest density and mosaic of grassland, savanna, ribbons of riparian vege- diversity of migratory birds compared to other habitats in tation and small patches of woods. The wooded habi- the region. In addition, we found the highest numbers for tats, in particular, can support an abundance of over half of the common migratory species. Despite the migratory birds (Powell et al., 1992; Warkentin et al., high degree of leaf loss during the late winter, acacia 1995). One possible strategy for increasing habitat for groves do not experience greater declines in insectivorous migratory birds is the promotion of silvopastoral sys- migratory bird populations than other local habitats. tems which integrate tree management with cattle pro- Color-marked individuals of canopy species had a strong duction on grazing lands. tendency to remain resident within a single acacia grove During several years of censusing birds on the Car- throughout the winter. Management of native acacias on ibbean slope of Chiapas, we discovered that managed subtropical rangelands for wood products, fodder, and soil patches of Acacia pennatula (Schlecht & Cham.) Benth improvement would probably directly and indirectly bene- support particularly high densities of migratory birds. -

Wildland Fire in Ecosystems: Effects of Fire on Fauna
United States Department of Agriculture Wildland Fire in Forest Service Rocky Mountain Ecosystems Research Station General Technical Report RMRS-GTR-42- volume 1 Effects of Fire on Fauna January 2000 Abstract _____________________________________ Smith, Jane Kapler, ed. 2000. Wildland fire in ecosystems: effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 83 p. Fires affect animals mainly through effects on their habitat. Fires often cause short-term increases in wildlife foods that contribute to increases in populations of some animals. These increases are moderated by the animals’ ability to thrive in the altered, often simplified, structure of the postfire environment. The extent of fire effects on animal communities generally depends on the extent of change in habitat structure and species composition caused by fire. Stand-replacement fires usually cause greater changes in the faunal communities of forests than in those of grasslands. Within forests, stand- replacement fires usually alter the animal community more dramatically than understory fires. Animal species are adapted to survive the pattern of fire frequency, season, size, severity, and uniformity that characterized their habitat in presettlement times. When fire frequency increases or decreases substantially or fire severity changes from presettlement patterns, habitat for many animal species declines. Keywords: fire effects, fire management, fire regime, habitat, succession, wildlife The volumes in “The Rainbow Series” will be published during the year 2000. To order, check the box or boxes below, fill in the address form, and send to the mailing address listed below. -

Appendix S1. List of the 719 Bird Species Distributed Within Neotropical Seasonally Dry Forests (NSDF) Considered in This Study
Appendix S1. List of the 719 bird species distributed within Neotropical seasonally dry forests (NSDF) considered in this study. Information about the number of occurrences records and bioclimatic variables set used for model, as well as the values of ROC- Partial test and IUCN category are provide directly for each species in the table. bio 01 bio 02 bio 03 bio 04 bio 05 bio 06 bio 07 bio 08 bio 09 bio 10 bio 11 bio 12 bio 13 bio 14 bio 15 bio 16 bio 17 bio 18 bio 19 Order Family Genera Species name English nameEnglish records (5km) IUCN IUCN category Associated NDF to ROC-Partial values Number Number of presence ACCIPITRIFORMES ACCIPITRIDAE Accipiter (Vieillot, 1816) Accipiter bicolor (Vieillot, 1807) Bicolored Hawk LC 1778 1.40 + 0.02 Accipiter chionogaster (Kaup, 1852) White-breasted Hawk NoData 11 p * Accipiter cooperii (Bonaparte, 1828) Cooper's Hawk LC x 192 1.39 ± 0.06 Accipiter gundlachi Lawrence, 1860 Gundlach's Hawk EN 138 1.14 ± 0.13 Accipiter striatus Vieillot, 1807 Sharp-shinned Hawk LC 1588 1.85 ± 0.05 Accipiter ventralis Sclater, PL, 1866 Plain-breasted Hawk LC 23 1.69 ± 0.00 Busarellus (Lesson, 1843) Busarellus nigricollis (Latham, 1790) Black-collared Hawk LC 1822 1.51 ± 0.03 Buteo (Lacepede, 1799) Buteo brachyurus Vieillot, 1816 Short-tailed Hawk LC 4546 1.48 ± 0.01 Buteo jamaicensis (Gmelin, JF, 1788) Red-tailed Hawk LC 551 1.36 ± 0.05 Buteo nitidus (Latham, 1790) Grey-lined Hawk LC 1516 1.42 ± 0.03 Buteogallus (Lesson, 1830) Buteogallus anthracinus (Deppe, 1830) Common Black Hawk LC x 3224 1.52 ± 0.02 Buteogallus gundlachii (Cabanis, 1855) Cuban Black Hawk NT x 185 1.28 ± 0.10 Buteogallus meridionalis (Latham, 1790) Savanna Hawk LC x 2900 1.45 ± 0.02 Buteogallus urubitinga (Gmelin, 1788) Great Black Hawk LC 2927 1.38 ± 0.02 Chondrohierax (Lesson, 1843) Chondrohierax uncinatus (Temminck, 1822) Hook-billed Kite LC 1746 1.46 ± 0.03 Circus (Lacépède, 1799) Circus buffoni (Gmelin, JF, 1788) Long-winged Harrier LC 1270 1.61 ± 0.03 Elanus (Savigny, 1809) Document downloaded from http://www.elsevier.es, day 29/09/2021. -
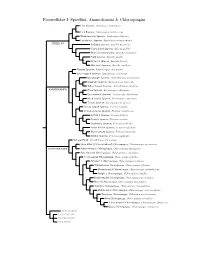
Passerellidae Species Tree
Passerellidae I: Spizellini, Ammodramini & Chlorospingini Lark Sparrow, Chondestes grammacus Lark Bunting, Calamospiza melanocorys Black-throated Sparrow, Amphispiza bilineata Five-striped Sparrow, Amphispiza quinquestriata SPIZELLINI Chipping Sparrow, Spizella passerina Clay-colored Sparrow, Spizella pallida Black-chinned Sparrow, Spizella atrogularis Field Sparrow, Spizella pusilla Brewer’s Sparrow, Spizella breweri Worthen’s Sparrow, Spizella wortheni Tumbes Sparrow, Rhynchospiza stolzmanni Stripe-capped Sparrow, Rhynchospiza strigiceps Grasshopper Sparrow, Ammodramus savannarum Grassland Sparrow, Ammodramus humeralis Yellow-browed Sparrow, Ammodramus aurifrons AMMODRAMINI Olive Sparrow, Arremonops rufivirgatus Green-backed Sparrow, Arremonops chloronotus Black-striped Sparrow, Arremonops conirostris Tocuyo Sparrow, Arremonops tocuyensis Rufous-winged Sparrow, Peucaea carpalis Cinnamon-tailed Sparrow, Peucaea sumichrasti Botteri’s Sparrow, Peucaea botterii Cassin’s Sparrow, Peucaea cassinii Bachman’s Sparrow, Peucaea aestivalis Stripe-headed Sparrow, Peucaea ruficauda Black-chested Sparrow, Peucaea humeralis Bridled Sparrow, Peucaea mystacalis Tanager Finch, Oreothraupis arremonops Short-billed (Yellow-whiskered) Chlorospingus, Chlorospingus parvirostris CHLOROSPINGINI Yellow-throated Chlorospingus, Chlorospingus flavigularis Ashy-throated Chlorospingus, Chlorospingus canigularis Sooty-capped Chlorospingus, Chlorospingus pileatus Wetmore’s Chlorospingus, Chlorospingus wetmorei White-fronted Chlorospingus, Chlorospingus albifrons Brown-headed -

Taxonomic Round-Up
Cotinga 17 Taxonom ic Round-up A new species of piha from the flycatcher (Tyrannidae: ruficeps by the río Marañón, and Colombian Andes Myiopagis) from eastern can be distinguished from the Andrés Cuervo and colleagues Ecuador and eastern Peru. latter form by virtue of its have discovered a new species of Wilson Bull. 112: 305–312. different song, facial pattern and piha in the Cordillera Central of belly coloration. Colombia. Lipaugus weberi, the A new tyrannulet from the • Johnson, N. K. & Jones, R. E. Chestnut-capped Piha, is closely white-sand forests of Peru (2001) A new species of tody- related to Dusky Piha L. José Alonso and Bret Whitney tyrant (Tyrannidae: fuscocinereus, a much more have described a new Zimmerius Poecilotriccus) from northern widespread Andean species. It is, tyrannulet from poorly drained Peru. Auk 118: 334–341. however, much smaller with a white-sand forest in the vicinity chestnut-brown crown, yellow of Iquitos, dpto. Loreto, Peru. Geographic variation in Suiriri orbital ring, two modified Zimmerius villarejoi, the Mishana Flycatcher primaries in the male, and unique Tyrannulet, is closely related to Floyd Hayes has recently vocalisations. The new species is the syntopic Slender-footed analysed geographic variation restricted to a very narrow belt of Tyrannulet Z. gracilipes, but within the three distinct taxa super-humid premontane forest, a differs in its concolorous described among the Suiriri habitat now highly fragmented upperparts, lack of whitish Flycatcher complex, namely within its range. feathering in the superciliary, Suiriri suiriri suiriri (of the • Cuervo, A. M., Salaman, P. G. loral and frontal regions, and by Chaco/Pampas), S.