Wo 2007/075896 A2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Xenoport Hurt by Solzira NDA Withdrawal
November 11, 2008 Xenoport hurt by Solzira NDA withdrawal Evaluate Vantage News that Xenoport and partner GlaxoSmithKline’s recent application for restless legs syndrome (RLS) drug Solzira will have to be withdrawn, with the FDA requesting that data from a single trial be re-formatted, caused Xenoport’s shares to fall 13% yesterday to a new 18-month low of $34.44. With a potential 4-6 month delay to approval and the postponement of a $23m milestone that Xenoport was due to receive from Glaxo on the FDA’s acceptance of Solzira’s NDA, senior executives at the specialty US company could be forgiven for feeling somewhat aggrieved with Glaxo for this apparent oversight, given the pharma giant’s supposed experience and expertise in regulatory filings. The setback to Solzira and resulting slump in Xenoport’s share price suggests that shareholders who have not fled the stock will be even more desperate for positive phase IIb data for GERD treatment, XP19986 (Event - XenoPort looking for end of year trial lift, October 10, 2008). Formatting issues Avoiding these kinds of regulatory pitfalls, after all the cost and hard work involved in taking a drug through clinical development, is becoming an increasingly important factor behind a company’s decision to sign up a big pharma partner in today’s tougher regulatory environment. Both companies were naturally keen to stress that the NDA withdrawal has nothing to do with the content of the filing, which probably makes the rejection on a technicality all the more frustrating for Xenoport. Whilst the FDA requested the data from one particular study to be re-formatted, Glaxo has decided to review the formatting of other data sets in the application, presumably to ensure there can be no further grounds for rejection. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Stems for Nonproprietary Drug Names
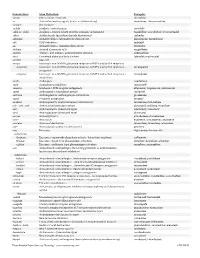
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Serotonin Dopamine Antagonists)
NewNew DevelopmentsDevelopments inin thethe ResearchResearch andand TreatmentTreatment ofof SchizophreniaSchizophrenia StephenStephen M.M. Stahl,Stahl, MD,MD, PhDPhD Adjunct Professor, Department of Psychiatry, University of California, San Diego School of Medicine Sponsored by Neuroscience Education Institute Additionally sponsored by American Society for the Advancement of Pharmacotherapy This activity is supported by educational grants from AstraZeneca Pharmaceuticals LP; Cephalon, Inc.; and Shire Pharmaceuticals Inc. with additional support from Alkermes, Inc.; Eli Lilly and Company; Jazz Pharmaceuticals, Inc.; and Sepracor Inc. Copyright © 2007 Neuroscience Education Institute. All rights reserved. Learning Objectives After completion of this lecture you should be able to: • Evaluate evidence about the function and efficacy of the newest antipsychotics • Describe the rationale and present state of pharmacogenetics research • Discuss goals and challenges of pharmacogenetics in psychiatry Copyright © 2008 Neuroscience Education Institute. All rights reserved. Overview: Four Bedtime Stories • A Tale of Serotonin Antagonism • The Dopamine Partial Agonism Story • Pharmacogenetics and the Genes • Once Upon a Glutamate Receptor Copyright © 2008 Neuroscience Education Institute. All rights reserved. Overview: Four Bedtime Stories • A Tale of Serotonin Antagonism • The Dopamine Partial Agonism Story • Pharmacogenetics and the Genes • Once Upon a Glutamate Receptor Copyright © 2008 Neuroscience Education Institute. All rights reserved. What -

2009 Paris, France the Movement Disorder Society’S 13Th International Congress of Parkinson’S Disease and Movement Disorders
FINAL PROGRAM The Movement Disorder Society’s 13th International Congress OF PARKINSon’S DISEASE AND MOVEMENT DISORDERS JUNE 7-11, 2009 Paris, France The Movement Disorder Society’s 13th International Congress of Parkinson’s Disease and Movement Disorders Claiming CME Credit To claim CME credit for your participation in the MDS 13th International Congress of Parkinson’s Disease and Movement Disorders, International Congress participants must complete and submit an online CME Request Form. This Form will be available beginning June 10. Instructions for claiming credit: • After June 10, visit www.movementdisorders.org/congress/congress09/cme • Log in following the instructions on the page. You will need your International Congress Reference Number, located on the upper right of the Confirmation Sheet found in your registration packet. • Follow the on-screen instructions to claim CME Credit for the sessions you attended. • You may print your certificate from your home or office, or save it as a PDF for your records. Continuing Medical Education The Movement Disorder Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Credit Designation The Movement Disorder Society designates this educational activity for a maximum of 30.5 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. Non-CME Certificates of Attendance were included with your on- site registration packet. If you did not receive one, please e-mail [email protected] to request one. The Movement Disorder Society has sought accreditation from the European Accreditation Council for Continuing Medical Education (EACCME) to provide the following CME activity for medical specialists. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -
Downloaded from Survive Nursing | Survivenursing.Com V20110426
Generic Stem Stem Definition Examples -abine (see -arabine, -citabine) decitabine -ac Anti-inflammatory agents (acetic acid derivatives) bromfenac; dexpemedolac -acetam See -racetam -actide Synthetic corticotropins seractide -adol or -aldol- Analgesics (mixed opiate receptor agonists/ antagonists) tazadolene; spiradolene; levonantradol -adox Antibacterials (quinoline dioxide derivatives) carbadox -afenone Antiarrhythmics (propafenone derivatives) alprafenone; diprafenone -afil PDE5 inhibitors tadalafil -aj- Antiarrhythmics (ajmaline derivatives) lorajmine -aldrate Antacid aluminum salts magaldrate -algron Alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol Combined alpha and beta blockers labetalol; medroxalol -amivir (see -vir) -ampa Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) -ampanel Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; becampanel antagonists -ampator Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; forampator modulators -andr- Androgens nandrolone -anib Angiogenesis inhibitors semaxanib -anserin Serotonin 5-HT2 receptor antagonists altanserin; tropanserin; adatanserin -antel Anthelmintics (undefined group) carbantel -antrone Antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine Antineoplastics (arabinofuranosyl derivatives) fazarabine; fludarabine aril-, -aril, -aril- Antiviral (arildone derivatives) pleconaril; arildone; fosarilate -arit Antirheumatics (lobenzarit type) lobenzarit; clobuzarit -arol -

(12) Patent Application Publication (10) Pub. No.: US 2016/0220580 A1 Rubin Et Al
US 2016O220580A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0220580 A1 Rubin et al. (43) Pub. Date: Aug. 4, 2016 (54) SMALL MOLECULESCREENING FOR (60) Provisional application No. 61/497,708, filed on Jun. MOUSE SATELLITE CELL PROLIFERATION 16, 2011. (71) Applicant: PRESIDENT AND FELLOWS OF Publication Classification HARVARD COLLEGE, Cambridge, (51) Int. Cl. MA (US) A 6LX3/553 (2006.01) (72) Inventors: Lee L. Rubin, Wellesley, MA (US); A613 L/496 (2006.01) Amanda Gee, Alexandria, VA (US); A613 L/4439 (2006.01) Amy J. Wagers, Cambridge, MA (US) A613 L/404 (2006.01) (52) U.S. Cl. CPC ............. A6 IK3I/553 (2013.01); A61 K3I/404 (21) Appl. No.: 15/012,656 (2013.01); A61 K3I/496 (2013.01); A61 K 31/4439 (2013.01) (22) Filed: Feb. 1, 2016 (57) ABSTRACT The invention provides methods for inducing, enhancing or Related U.S. Application Data increasing satellite cell proliferation, and an assay for screen (63) Continuation-in-part of application No. 14/126,716, ing for a candidate compound for inducing, enhancing or filed on Jun. 13, 2014, now Pat. No. 9.248,185, filed as increasing satellite cell proliferation. Also provided are meth application No. PCT/US2012/042964 on Jun. 18, ods for repairing or regenerating a damaged muscle tissue of 2012. a Subject. Patent Application Publication Aug. 4, 2016 Sheet 1 of 44 US 2016/0220580 A1 FIG. A Patent Application Publication Aug. 4, 2016 Sheet 2 of 44 US 2016/0220580 A1 FIG. C. FIG. 2A Patent Application Publication Aug. -

Novel Therapeutic Strategies in Parkinson's Disease
Novel therapeutic strategies in Parkinson’s disease Peter Klivenyi, Laszlo Vecsei To cite this version: Peter Klivenyi, Laszlo Vecsei. Novel therapeutic strategies in Parkinson’s disease. European Journal of Clinical Pharmacology, Springer Verlag, 2009, 66 (2), pp.119-125. 10.1007/s00228-009-0742-4. hal-00535002 HAL Id: hal-00535002 https://hal.archives-ouvertes.fr/hal-00535002 Submitted on 11 Nov 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Eur J Clin Pharmacol (2010) 66:119–125 DOI 10.1007/s00228-009-0742-4 REVIEW ARTICLE Novel therapeutic strategies in Parkinson’s disease Peter Klivenyi & Laszlo Vecsei Received: 30 June 2009 /Accepted: 30 September 2009 /Published online: 16 October 2009 # Springer-Verlag 2009 Introduction producing symptomatic benefit. However, in the subse- quent human clinical trials, many of them failed to produce Parkinson’s disease (PD) is a neurodegenerative disorder the same efficacy as seen in preclinical studies. These characterized by resting tremor, rigidity, and bradykinesia. failures are not necessarily an indication of drug failure or The pathological processes begin years/decades before the drug action but may be related to inadequate administration, first motor symptoms are observed. -

OMED 17 PHILADELPHIA, PENNSYLVANIA 29.5 Category 1-A CME Credits Anticipated
® OCTOBER 7 - 10 OMED 17 PHILADELPHIA, PENNSYLVANIA 29.5 Category 1-A CME credits anticipated ACOFP / AOA’s 122nd Annual Osteopathic Medical Conference & Exposition Joint Session with ACOFP and Cleveland Clinic: Managing Chronic Disease Parkinson's Disease: Early Warning Signs, When to Refer Hubert Fernandez, MD The American College of Osteopathic Family Physicians is accredited by the American Osteopathic Association Council to sponsor continuing medical education for osteopathic physicians. The American College of Osteopathic Family Physicians designates the lectures and workshops for Category 1-A credits on an hour-for-hour basis, pending approval by the AOA CCME, ACOFP is not responsible for the content. 10/5/2017 Early (and Late) Signs of PD: When to Refer Hubert H. Fernandez, MD, FAAN, FANA Professor of Medicine (Neurology) Cleveland Clinic Lerner College of Medicine Center Director Center for Neurological Restoration, Cleveland Clinic Disclosures Consulting: AbbVie, Acadia, Eli Lilly, Indus, Ipsen, Merz, Novartis, Pfizer, Sunovion, Voyager Independent Contractor (including contracted research): Acordia, Biogen, Kyowa Hakko, Michael J Fox Foundation, NIH/NINDS, Parkinson Study Group, Rhythm, Teva Teaching and Speaking: Medscape, Vindico Education 1 10/5/2017 Who gets PD? . Mean age of onset: 60-70, M>F . Affects 0.3% of the population and 1% of those older than 60 . Over 1.5 million people in North America affected Nutt JG and Wooten GF. New Engl J Med 2005;353(10): 1021-1027 PD Motor Symptoms 2 10/5/2017 The Parkinson Family Essential Tremor Progressive Drugs Multi- Vascular Supranuclear System Palsy Atrophy Idiopathic Toxins Parkinson’s Parkinson- Other Secondary Disease plus Encephalitis Lewy Cortico Body Basal Alzheimer’s Other Disease Degeneration Disease A Brief History of Our Understanding of PD 1917 2017 paralysis 1817 agitans Goetz CG. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

New Information of Dopaminergic Agents Based on Quantum Chemistry Calculations Guillermo Goode‑Romero1*, Ulrika Winnberg2, Laura Domínguez1, Ilich A
www.nature.com/scientificreports OPEN New information of dopaminergic agents based on quantum chemistry calculations Guillermo Goode‑Romero1*, Ulrika Winnberg2, Laura Domínguez1, Ilich A. Ibarra3, Rubicelia Vargas4, Elisabeth Winnberg5 & Ana Martínez6* Dopamine is an important neurotransmitter that plays a key role in a wide range of both locomotive and cognitive functions in humans. Disturbances on the dopaminergic system cause, among others, psychosis, Parkinson’s disease and Huntington’s disease. Antipsychotics are drugs that interact primarily with the dopamine receptors and are thus important for the control of psychosis and related disorders. These drugs function as agonists or antagonists and are classifed as such in the literature. However, there is still much to learn about the underlying mechanism of action of these drugs. The goal of this investigation is to analyze the intrinsic chemical reactivity, more specifcally, the electron donor–acceptor capacity of 217 molecules used as dopaminergic substances, particularly focusing on drugs used to treat psychosis. We analyzed 86 molecules categorized as agonists and 131 molecules classifed as antagonists, applying Density Functional Theory calculations. Results show that most of the agonists are electron donors, as is dopamine, whereas most of the antagonists are electron acceptors. Therefore, a new characterization based on the electron transfer capacity is proposed in this study. This new classifcation can guide the clinical decision‑making process based on the physiopathological knowledge of the dopaminergic diseases. During the second half of the last century, a movement referred to as the third revolution in psychiatry emerged, directly related to the development of new antipsychotic drugs for the treatment of psychosis.