Advanced Microwave Technology for Biodiesel Feedstock Processing Beatrice G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry and Physical Properties of Estolides
GRASAS Y ACEITES, 62 (1), ENERO-MARZO, 8-20, 2011, ISSN: 0017-3495 DOI: 10.3989/gya/010810 Chemistry and physical properties of estolides By Terry A. Isbell* United States Department of Agriculture, Agriculture Research Service, National Center for Agricultural Utilization Research, 1815 N. University St. Peoria, Illinois 61529 (*Corresponding author: [email protected]) RESUMEN capped estolides of oleic that have both good low temperature properties (pour point –5 to – 39oC) and good oxidative stability. Propiedades físicas y químicas de los estólidos Estolides from meadowfoam fatty acids do not have good low temperature properties but have been extensively used in Los estólidos son una familia de compuestos sintetizados cosmetics where they provide good moisturizing properties. a partir de aceites hidroxilados como los de ricino o lesquere- lla o mediante la condensación de ácidos grasos sobre el do- KEY-WORDS: Castor – Estolide – Lesquerella – Oleic ble enlace de un segundo ácido graso insaturado. Los estóli- acid – Physical Properties – Synthesis. dos de ricino y lesquerela se derivan tanto de sus triglicéridos como de sus ácidos grasos libres empleándose el residuo hi- droxilo para formar los ésteres estólidos de los mismos. Los triglicéridos estólidos tienen puntos de fluidez crítica de entre 1. INTRODUCTION 9 y -36ºC y baja estabilidad, con tiempos de oxidación en reci- piente vacío a presión (RPVOT) de entre 29 y 52 minutos in- Estolides are natural and synthetic compounds cluso con la adición de un 1% de una mezcla antioxidante a derived from fats and oils. The estolide structure las muestras. Estas propiedades contrastan con las de los es- is identified by the secondary ester linkage of one tólidos de ácido lesquerólico y ricinoleico, que poseen puntos fatty acyl molecule to the alkyl backbone of another críticos de fluidez mucho más bajos (de -36 a -54). -
![[ Agr. Biol. Chem., Vol. 29, No. 2, P. 111-116, 1965] Glyceride Structure](https://docslib.b-cdn.net/cover/4532/agr-biol-chem-vol-29-no-2-p-111-116-1965-glyceride-structure-284532.webp)
[ Agr. Biol. Chem., Vol. 29, No. 2, P. 111-116, 1965] Glyceride Structure
[ Agr. Biol. Chem., Vol. 29, No. 2, p. 111-116, 1965] Glyceride Structure and Biosynthesis of Natural Fats Part IV Biosynthetic Process of Triglycerides in Maturing Seed of Chinese Tallow Tree By Osamu HIRAYAMAand Shingo OHAMA Collegeof Agriculture,Kyoto Prefectural University, Kyoto ReceivedAugust 20, 1964 Changes in lipid classes and water-soluble components in maturing seed of Chinese tallow tree were examined. Rapid oil production occurred at three stages of seed maturity. Stillingia oil syntheses in seed kern proceeded more rapidly than stillingia tallow on the kern at early stage. Analytical data for components in immature seed suggest that the pathway of seed oil synthesis is the same as Kennedy's pathway. However, the appear ance of monoglycerides indicates the existence of a side pathway. From specific positional distribution of fatty acids in mono-, di-, and triglyceride from immature seed, synthetic process of glyceride structure was discussed. In previous paper,1) biosynthetic process of flowering, and sample number was given as shown triglycerides in maturing soybean seed has in Table 1. been investigated by measuring changes in Extraction and Fractionation of Lipids and the contents and composition of lipid and Water-soluble Components water-soluble components. A similar investi The seeds were extracted two times with hot gation was attemped on maturing seed of acetone under shaking. The extracts were combined,, Chinese tallow tree (Sapium sebiferum Roxb.) in and evaporated to dryness to obtain crude stillingia the present work. Chinese tallow tree ac- tallow. The fat was further refined by re-extraction with diethyl ether and evaporation. The residual seed cumulates solid fat (stillingia tallow) on the kern was crushed and homogenized with a mixture surface of seed kern, and liquid oil (stillingia of chloroform-methanol (2:1, v/v) using homogenizer. -

New Oilseed Crops on the Horizon!
New Oilseed Crops on the Horizon!: L. H. PRINCEN2 Fats and oils for food uses are now plentiful on a worldwide basis. Tallow, lard and fish oils, as well as vegetable oils, such as those derived from soybean, sun 1\ flower, palm, rapeseed, peanut and cottonseed, are often overproduced. Although many ofthese products are also used for industrial chemicals, they often are not [ ofthe most favorable composition for nonfood applications. A search for new oil 1\ seed crops with more advantageous oil composition has led to the development of l- excellent candidates that are now close to commercial acceptance. Among them are Crambe, Limnanthes, Vernonia, Sapium and Simmondsia. Other crops are at a much lower stage ofdevelopment but also have excellent potential. They include Cuphea, Foeniculum, Stokesia, Lesquerella and Lunaria. In this age ofsearching for renewable resources to replace petrochemicals and importedstrategic materials, c a well-organized research and development program on new oilseed crops could u soon result in American self-sufficiency for industrial oils andfatty acids. c Animal fats and vegetable oils make up an important part of the world's ag t ricultural production. Previous papers in this symposium have shown their eco a nomic impact from production through utilization. Although through the ages fats and oils have been produced mainly for food purposes, even centuries ago many also found use in such items as lubricants and greases, paints, soaps, lamp a oil and candles. Today, in the era ofpetrochemicals, fats and oils still playa major ( role in the production ofnonfood materials (Pryde, 1979). For example, in paints t and other coatings, where water-based latex systems have replaced much of the traditional vegetable oil-based paint market, approximately one-third ofthe bind ers used is still based on vegetable oils or their derivatives. -

A-Anions 221 Acetate 23, 106 Acetate-Malonate Pathway 24
Index a-anions 221 anti-block agent 218, 237 acetate 23, 106 antioxidants 84, 173, 175, 176 acetate-malonate pathway 24 anti-slip agent 218, 237 acetylenic acids 10, 11, 159 Apis mellifera 85 acetylenic intermediates 30 apricot oil 66, 67 acid anhydrides 214 arachidic acid 5, 6 acid chlorides 214 arachidonic acid 8,9, 29, 184 acidity 102 synthesis 31, 32 acidolysis 208,209, 213 Arachis hypogaea 67 ACP, see acyl carrier protein argentation tic 19 activated carbon 90 arsenites 18 acyl carrier protein 25 asci epic acid 7 acylation 47 Aspergillus niger 88, 97, 213 acylglycerols 36, 52 Aspergillus oryzae 213 acylpyrrolidide 154 auricolic acid 13, 14 alchornoic acid 15 autoxidation 162-167 alcohols 217,238 avenasterol 84 alcoholysis 208, 209 avocado oil 67 alepramic acid 14 aziridines 202 aleprestic acid 14 alepric acid 14 babassu oil 88 aleprolic acid 14 beef tallow 72 aleprylic acid 14 beeswax 84 Aleurites fordii 10 behenic acid 5, 6 alkanoic acids, crystal structure 130 bentonitite 90 alkylbenzoxazole 154 benzyl-sn-glycerol 54 alkyl dimethyloxazole 154 benzylidene glycerol 48 alkyl ketene dimers (AKD) 215 Betapol 55, 214 alkyl polyglucoside 217 BHA, see butylated hydroxyanisole allenic acids 10, 11, 39 BHT, see butylated hydroxy toluene allenic epoxides 187 biodiesel 241 allylic bromination 194 biohydrogenation 161 alklylic hydroperoxides 162, 163, 165 biosynthesis 24, 44 almond oil 66, 67, 69 blackcurrant oil 64, 65 alternation 130 bleaching 89, 90 amides 218, 238 Bligh and Dyer method 101 amine oxides 219, 220, 237 BOB, see oleodibehenin -
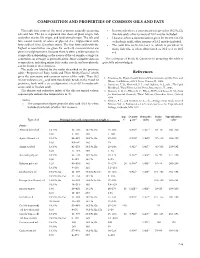
Composition and Properties of Common Oils and Fats References
COMPOSITION AND PROPERTIES OF COMMON OILS AND FaTS This table lists some of the most common naturally occurring • In some oils where a concentration is given for 18:2 9c,12c oils and fats . The list is separated into those of plant origin, fish (linoleic acid), other isomers of 18:2 may be included . and other marine life origin, and land animal origin . The oils and • Likewise, where a concentration is given for 18:3 9c,12c,15c fats consist mainly of esters of glycerol (i .e ., triglycerides) with (α-linolenic acid), other isomers of 18:3 may be included . fatty acids of 10 to 22 carbon atoms . The four fatty acids with the • The acid 20:5 6c,9c,12c,15c,17c, which is prevalent in highest concentration are given for each oil; concentrations are many fish oils, is often abbreviated as 20:5 ω-3 or 20:5 given in weight percent . Because there is often a wide variation in n-3 . composition depending on the source of the oil sample, a range (or sometimes an average) is generally given . More complete data on The assistance of Frank D . Gunstone in preparing this table is composition, including minor fatty acids, sterols, and tocopherols, gratefully acknowledged . can be found in the references . The acids are labeled by the codes described in the previous table, “Properties of Fatty Acids and Their Methyl Esters,” which References gives the systematic and common names of the acids . Thus 18:2 1 . Firestone, D ., Physical and Chemical Characteristics of Oils, Fats, and 9c,12c indicates a C18 acid with two double bonds in the 9 and 12 Waxes, 2nd Edition, AOCS Press, Urbana, IL, 2006 . -

Vegetable Oil Paints
Vegetable Oil Paints L. L. CARRICK, University of Michigan, Ann Arbor, Michigan HE Egyptians recognized the inedicinal proper- table waxes, pitches, or the oils from wild nuts or T ties of linseed oil but were unacquainted with its seeds. The early use of raw walnut, linseed, poppy drying properties. Ludius used oil in encaustic seed, olive, or hcmpseed oils has been noted together paints during the reign of Agustus (63 B.C.-14 A.D,). with the advent of their refined and bleached modifi- Vitruvius, during the same period, prepared a var- cation. Chinese tung oil, according to D'Incarville, nish from Punic wax and a little oil. Dioseorides 1735, was first a common adulterant of Chinese lac- (69-30 B.C.) recognized the dispersion properties of quers and did not commercially replace linseed oil in oils for natural resins. Pliny the author (A.D. 23-70) many varnishes in the United States until 1900. makes no mention of the use of oil by artists or Enamels were unknown before the beginning of the painters. Lucanus (A.D. 37-68) mentioned the use present century. Prior to this time it was common of oil to improve the working characteristics of color practice to apply a flat oil paint of the desired color for paints. Galen (A.D. 131-200) coagulated drying and follow it with a coat of clear varnish to obtain oils, such as hempseed, nut, and linseed oils by means the needed gloss. About this time it was found that of litharge, white lead, and umber. Actius (A.D. -
Iiihhhhhhhhhhh
IIIHHHHHHHHHHH US005210241A United States Patent (19) 11 Patent Number: 5,210,241 Lin (45) Date of Patent: May 11, 1993 (54) PROCESS FOR PREPARING COCOA 56) References Cited BUTTEREQUIVALENT FROM U.S. PATENT DOCUMENTS SEM-REFINED NONTOXC CHINESE VEGETABLE TALLOW 3,093,480 6/1963 Arnold ............................. 260/404.5 4,127,597 11/1978 Craig et al. ...................... 260/404.5 76 Inventor: Yitian Lin, 441 Guang Fu Xi Lu, FOREIGN PATENT DOCUMENTS Shanghai, China 918 2/1963 Japan ................................ 260/428.5 897841 1/1982 U.S.S.R. ........................... 260/428.5 (21) Appl. No.: 815,605 Primary Examiner-Joseo G. Dees Assistant Examiner-Joseph M. Conrad (22 Filed: Jan. 3, 1992 Attorney, Agent, or Firm-Pennie & Edmonds 57 ABSTRACT Related U.S. Application Data This invention relates to a process of preparing Cocoa 63 Continuation of Ser. No. 388,770, Aug. 2, 1989, aban Butter Equivalent (CBE) from Chinese Vegetable Tal doned, which is a continuation-in-part of Ser. No. low (CVT) and to the product prepared. Crude CVT is 2,906, Jan. 13, 1987, abandoned. first subjected to a semi-refining process comprising an alkali treatment, a water wash and an adsorption step. The semi-refined CVT is subjected to a single step frac (30) Foreign Application Priority Data tional crystallization under controlled conditions. After Jan. 16, 1986 CN China ................................. 86100502 removal of the solvent from the crude CBE found in the mother liquor after filtration, the crude CBE is sub 51 Int. Cl. ......................... C07C 1/00; C07B 51/43 jected to a steam distillation process, addition of antioxi 52 U.S.C. -

The Chinese Tallow Tree
The Chinese Tallow Tree G. A. Breitenbeck, Prof. School of Plant, Environmental and Soil Sciences, LSU Baton Rouge, LA [email protected] Copyright 2009 Photos by G. A Breitenbeck Tallow tree’s potential: • Grows on land unsuited for row crop agriculture • Seeds can produce more than 20 times the oil/ac as soybeans • Has few insect or disease pests • Perennial that can conserve soil/water • Can be harvested within 3- 5 yrs The Tallow tree is a fast growing species that produces abundant quantities of seed rich in lipids suitable for the production of biodiesel and other uses. Because of its high seed yields, this perennial crop has legitimate potential to supply the biodiesel industry with critical feedstock at low-cost. Tallow trees readily adapt to soils too infertile, wet or saline for profitable agriculture and therefore commercial production will not compete with food crops for limited land resources. Duke (1998) states that in managed systems, CTT is capable of producing seed yields of 14,000 kg/ha (12,500 lbs/ac) containing 2,600 kg oil (~300 gal) and 2,800 kg tallow (~180 gal). Scheld et al (1980, 1984) reported yields of 4,000 to 10,000 kg/ha, and cite estimates of 1050 gal/ac per year as a sustained energy yield. Observations in Louisiana confirm that exceptional naturalized trees growing in favorable environments are also capable of astonishing yields. A moderate sized tree covering 34 sq. yards produced 68 lbs of seed (Breitenbeck 2009). Some older trees have produced more than a 100 lbs of seed. -

Feedstock and Biodiesel Characteristics Report
Feedstock and Biodiesel Characteristics Report By Shannon D. Sanford*, James Matthew White, Parag S. Shah, Claudia Wee, Marlen A. Valverde, and Glen R. Meier Publication Date: November 17th, 2009 Renewable Energy Group® 416 S. Bell Avenue P.O. Box 888 Ames, IA 50010-0888 * Corresponding author. Tel.: +1 515 239 8175. E-mail address: [email protected] (Shannon Sanford). Please cite this article in press as: Sanford, S.D., et al., “Feedstock and Biodiesel Characteristics Report,” Renewable Energy Group, Inc., www.regfuel.com (2009). Table of Contents 1 Executive Summary .................................................................................................. 5 2 Disclaimer .................................................................................................................. 6 3 Feedstock Sources ..................................................................................................... 7 4 Feedstock Testing .................................................................................................... 11 4.1 Moisture ............................................................................................................ 11 4.2 Free Fatty Acid (FFA) ...................................................................................... 13 4.3 Kinematic Viscosity .......................................................................................... 16 4.4 FAC Color ......................................................................................................... 18 4.5 Saponification -

Protabase Record Display Triadica Sebifera
Protabase Record display www.prota.org Triadica sebifera (L.) Small Protologue Florida trees: 59 (1913). Family Euphorbiaceae Chromosome number 2n = 36 Synonyms Stillingia sebifera (L.) Michx. (1803), Sapium sebiferum (L.) Roxb. (1832). Vernacular names Chinese tallow tree, candleberry tree, popcorn tree (En). Boiré, arbre à suif (Fr). Árvore do sebo, pau do sebo (Po). Origin and geographic distribution Triadica sebifera is native to China and Japan, where it is cultivated, but more widely in former times than at present. It was widely introduced as an ornamental tree in the tropics and subtropics, e.g. in northern India, Pakistan, the southern United States and around the Black Sea. In many of these areas it has become naturalized and sometimes weedy. It has occasionally been planted in tropical Africa, where it occurs from Sudan to South Africa. Uses The fruit of Triadica sebifera contains two types of fat: the white, fleshy outer seed coat (sarcotesta) yields a fat known as ‘Chinese vegetable tallow’ or ‘pi-yu’ in trade, while the seed kernel yields a drying oil called ‘stillingia oil’ or ‘ting-yu’ in trade. Chinese vegetable tallow is widely used in China for edible purposes, as a substitute for animal tallow and for lighting. Candles made by mixing 10 parts Chinese vegetable tallow with 3 parts white insect wax are reputed to remain pure white for any length of time and to burn with a clear bright flame without smell or smoke. Elsewhere, Chinese vegetable tallow is used to make soap, as a substitute for cocoa butter and to increase the consistence of soft edible fats. -

Some Observations on the Composition of Chinese Lacquer
Studies in Conservation ISSN: 0039-3630 (Print) 2047-0584 (Online) Journal homepage: http://www.tandfonline.com/loi/ysic20 Some observations on the composition of Chinese lacquer Arlen Heginbotham, Julie Chang (張倚竹), Herant Khanjian & Michael R. Schilling To cite this article: Arlen Heginbotham, Julie Chang (張倚竹), Herant Khanjian & Michael R. Schilling (2016) Some observations on the composition of Chinese lacquer, Studies in Conservation, 61:sup3, 28-37, DOI: 10.1080/00393630.2016.1230979 To link to this article: https://doi.org/10.1080/00393630.2016.1230979 © 2016 The J. Paul Getty Trust. Published online: 15 Dec 2016. Submit your article to this journal Article views: 946 View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ysic20 Supplementary issue paper Some observations on the composition of Chinese lacquer Arlen Heginbotham1, Julie Chang (張倚竹)2, Herant Khanjian3, Michael R. Schilling3 1The J. Paul Getty Museum, Los Angeles, CA, USA, 2University College London, UK, 3The Getty Conservation Institute, Los Angeles, CA, USA This paper summarizes the various information that has been gathered in recent years at the J. Paul Getty Museum and Getty Conservation Institute with regard to the organic constituents of Chinese lacquer formulations. While this summary of materials is by no means comprehensive or complete, it captures the current state of the authors’ knowledge, with the information itself and the bibliography intended to serve as a useful foundation from which further research may proceed. Considerable advances have been made in the last decade in the technique of pyrolysis-gas chromatography–mass spectrometry with thermally assisted hydrolysis and methylation using tetramethylammonium hydroxide and in subsequent data interpretation methodologies. -

Parameterization of Microwave Assisted Oil Extraction and Its Transesterification to Biodiesel Akanksha V
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2010 Parameterization of microwave assisted oil extraction and its transesterification to biodiesel Akanksha V. Kanitkar Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Engineering Commons Recommended Citation Kanitkar, Akanksha V., "Parameterization of microwave assisted oil extraction and its transesterification to biodiesel" (2010). LSU Master's Theses. 4126. https://digitalcommons.lsu.edu/gradschool_theses/4126 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. PARAMETERIZATION OF MICROWAVE ASSISTED OIL EXTRACTION AND ITS TRANSESTERIFICATION TO BIODIESEL A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in Biological and Agricultural Engineering in The Department of Biological & Agricultural Engineering by Akanksha V. Kanitkar B.Tech., University Department of Chemical Technology, 2007 May 2010 DEDICATION ♥ To my beloved parents without whom I would not have come so far... ii ACKNOWLEDGMENTS I would like to thank the Almighty for his blessings towards achieving this goal in my life and my family for their encouragement and unconditional support. Mom and Dad, I owe all my success to you. I would like to express my gratitude towards my parents, Mrs.