Bio 215 Course Title: General Biochemistry Laboratory I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experiment 5: Molar Volume of a Gas (Mg + Hcl)
1 CHEM 30A EXPERIMENT 5: MOLAR VOLUME OF A GAS (MG + HCL) Learning Outcomes Upon completion of this lab, the student will be able to: 1) Demonstrate a single replacement reaction. 2) Calculate the molar volume of a gas at STP using experimental data. 3) Calculate the molar mass of a metal using experimental data. Introduction Metals that are above hydrogen in the activity series will displace hydrogen from an acid and produce hydrogen gas. Magnesium is an example of a metal that is more active than hydrogen in the activity series. The reaction between magnesium metal and aqueous hydrochloric acid is an example of a single replacement reaction (a type of redox reaction). The chemical equation for this reaction is shown below: Mg(s) + 2HCl(aq) è MgCl2(aq) + H2(g) Equation 1 When the reaction between the metal and the acid is conducted in a eudiometer, the volume of the hydrogen gas produced can be easily determined. In the experiment described below magnesium metal will be reacted with an excess of hydrochloric acid and the volume of hydrogen gas produced at the experimental conditions will be determined. According to Avogadro’s law, the volume of one mole of any gas at Standard Temperature and Pressure (STP = 273 K and 1 atm) is 22.4 L. Two important Gas Laws are required in order to convert the experimentally determined volume of hydrogen gas to that at STP. 1. Dalton’s law of partial pressures. 2. Combined gas law. Dalton’s Law of Partial Pressures According to Dalton’s law of partial pressures in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. -

Wo 2007/086935 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 2 August 2007 (02.08.2007) WO 2007/086935 A2 (51) International Patent Classification: Not classified (US). MYERS, Eugene, W. [-/US]; 20 Commercial Street, Branford, CT 06405 (US). SIMPSON, John, (21) International Application Number: W. [US/US]; 23 Woodland Road, Suite B4, Madison, PCT/US2006/030235 CT 06443 (US). VOLKMER, Greg, A. [-/US]; 20 Commercial Street, Branford, CT 06405 (US). (22) International Filing Date: 1 August 2006 (01.08.2006) (74) Agent: HOPKINS, Brian, P.; Mintz, Levin, Cohn, Ferris, (25) Filing Language: English Glovsky And Popeo, Rc, Chrysler Center, 666 Third Av enue, New York, NY 10017 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 11/195,254 1 August 2005 (01.08.2005) US AT,AU, AZ, BA, BB, BG, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, (71) Applicant (for all designated States except US): 454 LIFE GB, GD, GE, GH, GM, HN, HR, HU, ID, IL, IN, IS, JP, SCIENCES CORPORATION [US/US]; 20 Commercial KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, Street, Branford, CT 06405 (US). LU, LV,LY,MA, MD, MG, MK, MN, MW, MX, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, (72) Inventors; and SD, SE, SG, SK, SL, SM, SY, TJ, TM, TN, TR, TT, TZ, (75) Inventors/Applicants (for US only): MCDADE, Keith, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW E. -

22 Bull. Hist. Chem. 8 (1990)
22 Bull. Hist. Chem. 8 (1990) 15 nntn Cn r fr l 4 0 tllOt f th r h npntd nrpt ltd n th brr f th trl St f nnlvn n thr prt nd ntn ntl 1 At 1791 1 frn 7 p 17 AS nntn t h 3 At 179 brr Cpn f hldlph 8Gztt f th Untd Stt Wdnd 3 l 1793 h ntr nnnnt rprdd n W Ml "njn h Cht"Ch 1953 37-77 h pn pr dtd 1 l 179 nd nd b Gr Whntn th frt ptnt d n th Untd Stt S M ntr h rt US tnt" Ar rt Invnt hn 199 6(2 1- 19 ttrfld ttr fnjn h Arn hl- phl St l rntn 1951 pp 7 9 20h drl Gztt 1793 (20 Sptbr Qtd n Ml rfrn 1 1 W Ml rfrn 1 pp 7-75 William D. Williams is Professor of Chemistry at Harding r brt tr University, Searcy, Al? 72143. He collects and studies early American chemistry texts. Wyndham D. Miles. 24 Walker their British cultural heritage. To do this, they turned to the Avenue, Gaithersburg, MD 20877, is winner of the 1971 schools (3). This might explain why the Virginia assembly Dexter Award and is currently in the process of completing the took time in May, 1780 - during a period when their highest second volume of his biographical dictionary. "American priority was the threat of British invasion following the fall of Chemists and Chemical Engineers". Charleston - to charter the establishment of Transylvania Seminary, which would serve as a spearhead of learning in the wilderness (1). -

I I I I Executive Summary
I I I CONTAMINANTS IN FISH AND SEDIMENTS OF THE GREAT SWAMP NATIONAL WILDLIFE REFUGE, MORRIS COUNTY, NEW JERSEY: I A 10-YEAR FOLLOW-UP INVESTIGATION DEC ID# 9950003.1 I ECDM Catalog # 5050048 I I I Prepared for: U.S. Fish & Wildlife Service Northeast Region Division of Refuges and Wildlife I Hadley, Massachusetts I Prepared by: I U.S. Fish & Wildlife Service New Jersey Field Office I Plem,antville, New Jersey I I August 2005 I Project Biologist: Clay M. Stem Assistant Supervisor: Timothy J. Kubiak I Supen,isor: Clifford G. Day I I 2 I I I I EXECUTIVE SUMMARY Located in Morris County, New Jersey about 25 miles west of New York City's Time Square, the I U.S. Fish & Wildlife Service's (Service) Great Swamp National Wildlife Refuge (GSNWR) encompasses approximately 7,500 acres ::,fwhich 3,660 acres are designated and managed as a National Wilderness Area. The GSNWR's wetlands provide important ecological functions, I including floodwater attenuation, groundwater recharge, pollution abatement, wildlife habitat, as well as recreational benefits for the public. In many portions of the Great Swamp watershed, significant areas of native soils have been disturbed by development to the extent that the I original soil profiles no longer exist. Development-related activities such as grading, infilling, and compaction have adversely altered the native soil's infiltration capacity and runoff potential I and thereby have increased storm-water 11ediment loading into many of the watershed's streams. The purpose of this investigation is to conduct a 10-year follow-up to a 1988 investigation (USFWS 1991) characterizing ambient concentrations of metals, organochlorines, and polycyclic I aromatic hydrocarbons (P AHs) in GSNWR sediments, and metals and organochlorine in fish inhabiting the GSNWR. -

Determination of Calcium Metal in Calcium Cored Wire
Asian Journal of Chemistry; Vol. 25, No. 17 (2013), 9439-9441 http://dx.doi.org/10.14233/ajchem.2013.15017 Determination of Calcium Metal in Calcium Cored Wire 1,* 1 2 1 1 HUI DONG QIU , MEI HAN , BO ZHAO , GANG XU and WEI XIONG 1Department of Chemistry and Chemical Technology, Chong Qing University of Science and Technology, Chongqing 401331, P.R. China 2Shang Hai Entry-Exit Inspection and Quarantine Bureau, P.R. China *Corresponding author: Tel: +86 23 65023763; E-mail: [email protected] (Received: 24 December 2012; Accepted: 1 October 2013) AJC-14200 The calcium analysis method in cored wire products is mainly measuring the total calcium content, including calcium oxide and calcium carbonate. According to the chemical composition of calcium cored wire and the chemical property of the active calcium in it, the gas volumetric method has been established for the determination of calcium content in calcium cored wire and a set of analysis device is also designed. Sampling in the air directly, intercepting the inner filling material of samples, reacting with water for 10 min, using standard comparison method and then the accurate calcium content can be available. The recovery rate of the experimental method was 93-100 %, the standard deviation is less than 0.4 %. The method is suitable for the determination the calcium in the calcium cored wire and containing active calcium fractions. Key Words: Calcium cored wire, Active calcium, Gas volumetric method. INTRODUCTION various state of the calcium. However, because the sample is heterogeneous and the amount of the sample for instrumental 1-4 The calcium cored wire is used to purify the molten analysis is small, thus such sampling can not estimate charac- steel, modify the form of inclusions, improve the casting and teristics of the whole ones accurately, so there are flaws to use mechanical properties of the steel and also reduce the cost of the instrumental analysis to determine the calcium metal in steel. -

XXIX. on the Magnetic Susceptibilities of Hydrogen and Some Other Gases
Philosophical Magazine Series 6 ISSN: 1941-5982 (Print) 1941-5990 (Online) Journal homepage: http://www.tandfonline.com/loi/tphm17 XXIX. On the magnetic susceptibilities of hydrogen and some other gases Také Soné To cite this article: Také Soné (1920) XXIX. On the magnetic susceptibilities of hydrogen and some other gases , Philosophical Magazine Series 6, 39:231, 305-350, DOI: 10.1080/14786440308636042 To link to this article: http://dx.doi.org/10.1080/14786440308636042 Published online: 08 Apr 2009. Submit your article to this journal Article views: 6 View related articles Citing articles: 16 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=6phm20 Download by: [RMIT University Library] Date: 20 June 2016, At: 12:34 E 3o5 XXIX. On the MAgnetic Susceptibilities of llydrogen uud some otl~er Gases. /33/TAK~; S0~; *. INDEX TO SECTIONS. 1. INTRODUCTION. ~O. METI~OD OF 5LEASUREMENT. o. APPARATUS :FOR }IEASU14EIMF~NT. (a) Magnetic bMance. (b) Compressor and measuring tube. 4. PIt0CEDUI{E :FOR BIEASUREMENTS. (a) Adjustment of the measuring tube. (b) Determination of the mass. (c) Method of filling the measuring tube with gas. (d) Electromagnet. (e} Method of experiments. 5. Ain. 6. OXYGEN. ~. CARBON DIOXIDE. 8. NITROGEN. 9. ItYDI~0G~N. (a) Preparation of pure hydrogen gas. (b) Fillin~ the measuring tube with the gas. (e) YCesults el'magnetic measurement. (d} Purity of the hydrogen gas. 10. CONCLUDING REMARKS. wi. INTRODUCTION. N the electron theory of magnetism, it is assumed that I the magnetism is duo to electrons revolving about the positive nucleus in the atom; and hence the electronic structure of the atom has a very important bearing on its magnetic properties. -

A Study of Form and Content for a Laboratory Manual to Be Used by Students in General Chemistry Laboratory
Brigham Young University BYU ScholarsArchive Theses and Dissertations 1946-01-01 A study of form and content for a laboratory manual to be used by students in general chemistry laboratory Berne P. Broadbent Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd BYU ScholarsArchive Citation Broadbent, Berne P., "A study of form and content for a laboratory manual to be used by students in general chemistry laboratory" (1946). Theses and Dissertations. 8176. https://scholarsarchive.byu.edu/etd/8176 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. ?_(j;, . , i. ~ ~ (12 ' -8?5: 11% -· • A STUDY OF FOFM CONTENTFOR A LABORATORY -\ .AND MANUALTO BE USED BY STUDENTSIN GENERALCEEi/IISTRY LABORATORY' A THESIS SUBMITTEDTO \ THE DEPAR'.I3\t1Ell."'T OF CHEMISTRY OF ··! BRIGHAMYOUNG UNIVERSITY,. IN PARTIALFULFII.lllENT OF THEREQ,UIREMENTS·FOR THE DEGREE OF MASTEROF SCIENCE ... .,; . •·' .. ...• • .• . • "f ... ·.. .. ,. ·: :. !./:.•:-.:.lo>•.-,:... ... ... ..........• • • • p ,.. .,• • • ...• • . ~. ••,,. ................. :... ~•••,,.c • ..............• • • • • • .. f" ·~•-~-·"••• • • • ... • .., : :·.•··•:'"'•••:'"',. ·.-··.::· 147141 BY BERNEP. BROADBENT . " 1946 .,_ - ii \ ., This Thesis by Berne P.- Broadbent is accepted in 1ts P:esent form by the Departm·ent of Chem�stry as satisfying the Thesis requirement.for the degree of .J Master of Science • ,• . - .} .. iii PREF.ACE The constantly broadening field assigned to general chemistry demands that material be carefully selected and that ever increasing attention be given to preparing this material and presenting it_ to the student.- The following study was made to develop a laboratory manual that would increase the effectiveness of laboratory work. -
CHAPTER 15 the Laboratory
CHAPTER 15 The Laboratory These skills are usually tested on the SAT Subject Test in Chemistry. You should be able to . • Name, identify, and explain proper laboratory rules and procedures. • Identify and explain the proper use of laboratory equipment. • Use laboratory data and observations to make proper interpretations and conclusions. This chapter will review and strengthen these skills. Be sure to do the Practice Exercises at the end of the chapter. Laboratory setups vary from school to school depending on whether the lab is equipped with macro- or microscale equipment. Microlabs use specialized equipment that allows lab work to be done on a much smaller scale. The basic principles are the same as when using full-sized equipment, but microscale equipment lowers the cost of materials, results in less waste, and poses less danger. The examples in this book are of macroscale experiments. Along with learning to use microscale equipment, most labs require a student to learn how to use technological tools to assist in experiments. The most common are: Gravimetric balance with direct readings to thousandths of a gram instead of a triple-beam balance pH meters that give pH readings directly instead of using indicators Spectrophotometer, which measures the percentage of light transmitted at specific frequencies so that the molarity of a sample can be determined without doing a titration Computer-assisted labs that use probes to take readings, e.g., temperature and pressure, so that programs available for computers can print out a graph of the relationship of readings taken over time LABORATORY SAFETY RULES The Ten Commandments of Lab Safety The following is a summary of rules you should be well aware of in your own chemistry lab. -

(12) United States Patent (10) Patent No.: US 8,557,518 B2 Jovanovich Et Al
US008557518B2 (12) United States Patent (10) Patent No.: US 8,557,518 B2 Jovanovich et al. (45) Date of Patent: Oct. 15, 2013 (54) MCROFLUDIC AND NANOFLUIDC (56) References Cited DEVICES, SYSTEMS, AND APPLICATIONS U.S. PATENT DOCUMENTS (75) Inventors: Stevan Bogdan Jovanovich, Livermore, 3,190,310 A 6/1965 Honsinger CA (US); Iuliu I. Blaga, Fremont, CA 11/1967 Ando et al. (US); Michael Nguyen, San Jose, CA 3,352,643 A (US); William D. Nielsen, San Jose, CA (Continued) (US); Mattias Vangbo, Fremont, CA (US) FOREIGN PATENT DOCUMENTS CA 2433145 A1 5, 2002 (73) Assignee: IntegenX Inc., Pleasanton, CA (US) EP O459.241 B1 12, 1991 (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 109 days. Shaikh et all “A modular microfluidic archetecture for integrated (21) Appl. No.: 12/845,650 biochemical analysis”, PNAS, 2005, 102: 9745-9750.* (22) Filed: Jul. 28, 2010 (Continued) (65) Prior Publication Data Primary Examiner — Betty Forman US 2012/O1151.89 A1 May 10, 2012 (57) ABSTRACT Related U.S. Application Data The present invention discloses the integration of program (63) Continuation of application No. 12/526,015, filed as mable microfluidic circuits to achieve practical applications application No. PCT/US2008/053099 on Feb. 5, 2008. to process biochemical and chemical reactions and to inte grate these reactions. In some embodiments workflows for (60) Provisional application No. 60/899,630, filed on Feb. biochemical reactions or chemical workflows are combined. 5, 2007. Microvalves such as programmable microfluidic circuit with Y valves and flow through valves are disclosed. -

Inquiry-Based Laboratory Work in Chemistry
INQUIRY-BASED LABORATORY WORK IN CHEMISTRY TEACHER’S GUIDE Derek Cheung Department of Curriculum and Instruction The Chinese University of Hong Kong Inquiry-based Laboratory Work in Chemistry: Teacher’s Guide / Derek Cheung Copyright © 2006 by Quality Education Fund, Hong Kong All rights reserved. Published by the Department of Curriculum and Instruction, The Chinese University of Hong Kong. No part of this book may be reproduced in any manner whatsoever without written permission, except in the case of use as instructional material in a school by a teacher. Note: The material in this teacher’s guide is for information only. No matter which inquiry-based lab activity teachers choose to try out, they should always conduct risks assessment in advance and highlight safety awareness before the lab begins. While every effort has been made in the preparation of this teacher’s guide to assure its accuracy, the Chinese University of Hong Kong and Quality Education Fund assume no liability resulting from errors or omissions in this teacher’s guide. In no event will the Chinese University of Hong Kong or Quality Education Fund be liable to users for any incidental, consequential or indirect damages resulting from the use of the information contained in the teacher’s guide. ISBN 962-85523-0-9 Printed and bound by Potential Technology and Internet Ltd. Contents Preface iv Teachers’ Concerns about Inquiry-based Laboratory Work 1 Secondary 4 – 5 Guided Inquiries 1. How much sodium bicarbonate is in one effervescent tablet? 4 2. What is the rate of a lightstick reaction? 18 3. Does toothpaste protect teeth? 29 4. -
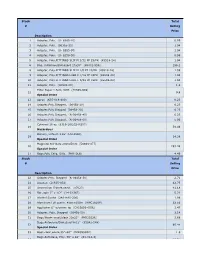
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Digestion of Algin by Pseudomonas Maltophilia and Pseudomonas Putida V
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 1980, p. 92-96 Vol. 39, No. 1 0099-2240/80/01-0092/05$02.00/0 Digestion of Algin by Pseudomonas maltophilia and Pseudomonas putida V. LYLE VON RIESEN Department ofMedical Microbiology, College ofMedicine, University of Nebraska Medical Center, Omaha, Nebraska 68105 Pseudomonas maltophilia and Pseudomonas putida were identified as al- ginolytic species. Two media used for demonstrating alginolytic activity are described. The applied aspects of the ability of these two species to digest algin are discussed. In a search for undescribed biochemical activ- Both media contained yeast extract (0.5%), algin (al- ities of nonfastidious, nonfermentative gram- ginic acid, sodium salt, no. A-7128; Sigma Chemical negative bacilli (NFB) which might be used to Co., St. Louis, Mo.) (1%), and bromothymol blue of these taxonomically (0.002%). Agar (0.5%) was used to form an algin-agar aid in the identification medium and animal charcoal (0.1%) to form an algin- troublesome organisms, a survey of a variety of charcoal medium. The media were prepared in 100- to complex polysaccharides as substrates was made 500-ml amounts in the following manner. Calculated by using a few strains of some well-recognized amounts of yeast extract and bromothymol blue (from species of NFB. When it was discovered that a 1% aqueous solution) were added to an appropriate some of the strains ofPseudomonas maltophilia volume of distilled water. This was then warmed on a showed evidence of an ability to hydrolyze algin magnetic stirrer-heater. When warm (40 to 60°C), (sodium alginate), an available collection of ap- algin and charcoal (algin-charcoal medium) or algin proximately 390 strains of NFB was examined and agar (algin-agar medium) were sprinkled into the of individual strains to digest vigorously stirred solution.