Crustacea: Decapoda: Atyidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 2, Number 1 (2011), pp. 23-32 © International Research Publication House http://www.irphouse.com A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India Siva Ranjanee S.1 and Mariapan N.2 1Department of Biotechnology, Vels University, Pallavaram, Chennai, India 2Senior Medical Writer, SIRO Clinpharm Pvt. Ltd., Thane (W) 400 607. India E-mail: [email protected], [email protected] Abstract Fresh water prawns are cultured widely around the world but little is known about the levels and patterns of genetic diversity. This paper reports the RAPD analysis of two species of fresh water prawns of Atydiae and Palaemonidiae family and the genus Macrobrachium and Caridina collected from Kanyakumari district. Specimens are identified using species-specific morphological characteristics. Morphological characters have limitations of describing intra specific genetic diversity as they are polygenic and expressions can be modified by the environment. The advent of molecular technique made possible not only the genetic analysis and also the study of evolutionary relationship. Molecular analysis of the morphologically identified species are done by extracting the DNA from the animal and concentration of DNA is measured using Nanodrop spectrophotometer at a wavelength of 280nm. Further analysis was performed with RAPD (Rapid Amplified Polymorphic DNA) a PCR (Polymerase Chain Reaction) based technique. It consist of genomic DNA, amplified with randomly constructed oligonucleotides. Specific quantity of extracted DNA was then amplified by multiplex PCR using random primers and 16S rRNA gene products was then analyzed using a bioanalyzer. -
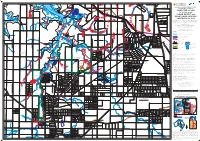
FLOOD EXTENT and PEAK FLOOD SURFACE CONTOURS for 2100
707500E 710000E 712500E 715000E 717500E 720000E 722500E 725000E 11 1 0 2 26 23 18 Middle Arm Road 19 22 BLACKMORE 17 19 27 25 18 1 NOONAMAH 2 19 ELIZABETH and BLACKMORE RIVER 23 3 5 24 20 CATCHMENTS — Sheet 2 25 4 20 4 COMPUTED 1% AEP RIVER 3 Aquiculture Farm Ponds 26 26 21 21 22 (1 in 100 year) 5 Middle Arm Boat Ramp 25 6 24 7 1 23 FLOOD EXTENT and 2 8 22 16 14 18 23 22 9 15 20 13 21 PEAK FLOOD SURFACE 24 10 1 19 12 17 20 23 CONTOURS for 2100 1 3 4 18 25 6 11 21 24 This map shows the Q100 flood and floodway extents caused by a 1% Annual 1.5 10 2 19 1.25 5 Exceedance Probability (AEP) Flood event over the Elizabeth and Blackmore 8600000N 8600000N 1.25 2 25 Keleson Road River Catchments. The extent of flooding shown on this map is indicative only, 17 Middle Arm Road 2 2 Strauss Field 26 hence, approximate. This map is available for sale from: 3 1.5 9 26 3 27 Land Information Centre, 1 1 8 27 3 1 2 Department of Lands, Planning and the Environment 3 1 3 7 16 1.5 1 3rd Floor NAB House, 71 Smith Street, Darwin, Northern Territory, 0800 28 29 29 Finn Road Finn 1.25 T: (08) 8995 5300 Email: [email protected] 6 30 2 28 31 GPO Box 1680, Darwin, Northern Territory, 0801. 3 31 15 4 5 14 27 33 This map is also available online at: 7 32 30 RIVER 6 7 8 10 35 www.nt.gov.au/floods http://nrmaps.nt.gov.au 9 13 11 12 8 26 32 Legend 2.5 4 5 25 34 3 24 6 Flood extent 3 7 24 8 23 Floodway, depth >2 metres (or velocity x depth > 1) 9 23 22 3 1.25 10 Peak flood surface contour, metres AHD 3 22 10.5 21 Creek channel / flow direction 21 20 Limit of flood mapping -

Species-Edition-Melanesian-Geo.Pdf
Nature Melanesian www.melanesiangeo.com Geo Tranquility 6 14 18 24 34 66 72 74 82 6 Herping the final frontier 42 Seahabitats and dugongs in the Lau Lagoon 10 Community-based response to protecting biodiversity in East 46 Herping the sunset islands Kwaio, Solomon Islands 50 Freshwater secrets Ocean 14 Leatherback turtle community monitoring 54 Freshwater hidden treasures 18 Monkey-faced bats and flying foxes 58 Choiseul Island: A biogeographic in the Western Solomon Islands stepping-stone for reptiles and amphibians of the Solomon Islands 22 The diversity and resilience of flying foxes to logging 64 Conservation Development 24 Feasibility studies for conserving 66 Chasing clouds Santa Cruz Ground-dove 72 Tetepare’s turtle rodeo and their 26 Network Building: Building a conservation effort network to meet local and national development aspirations in 74 Secrets of Tetepare Culture Western Province 76 Understanding plant & kastom 28 Local rangers undergo legal knowledge on Tetepare training 78 Grassroots approach to Marine 30 Propagation techniques for Tubi Management 34 Phantoms of the forest 82 Conservation in Solomon Islands: acts without actions 38 Choiseul Island: Protecting Mt Cover page The newly discovered Vangunu Maetambe to Kolombangara River Island endemic rat, Uromys vika. Image watershed credit: Velizar Simeonovski, Field Museum. wildernesssolomons.com WWW.MELANESIANGEO.COM | 3 Melanesian EDITORS NOTE Geo PRODUCTION TEAM Government Of Founder/Editor: Patrick Pikacha of the priority species listed in the Critical Ecosystem [email protected] Solomon Islands Hails Partnership Fund’s investment strategy for the East Assistant editor: Tamara Osborne Melanesian Islands. [email protected] Barana Community The Critical Ecosystem Partnership Fund (CEPF) Contributing editor: David Boseto [email protected] is designed to safeguard Earth’s most biologically rich Prepress layout: Patrick Pikacha Nature Park Initiative and threatened regions, known as biodiversity hotspots. -

Caridina Propinqua) Ecological Risk Screening Summary
Bengal Caridina (Caridina propinqua) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, July 2017 Revised, August 2017 Web Version, 11/17/2017 1 Native Range and Status in the United States Native Range From De Grave and Cai (2013): “The species is widespread from Bangladesh and India through to the Ryukyus and southwards to Singapore (Cai and Shokita 2006).” “Bangladesh; India (Orissa); Japan; Malaysia (Peninsular Malaysia); Philippines; Singapore; Sri Lanka; Thailand” Status in the United States This species has not been reported as introduced or established in the United States. This species is in trade in the U.S. From Bob’s Tropical Plants (2017): “Caridina cf. propinqua orange shrimp […] $2.75 tax excl.” “They are still relatively rare in the hobby, but are not difficult to maintain.” 1 Means of Introductions in the United States This species has not been reported as introduced or established in the United States. Remarks From GBIF (2016): “SYNONYMS Caridina blancoi Chace, 1997 Caridina hainanensis Liang & Yan, 1983” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2017): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Protostomia Superphylum Ecdysozoa Phylum Arthropoda Subphylum Crustacea Class Malacostraca Subclass Eumalacostraca Superorder Eucarida Order Decapoda Suborder Pleocyemata Infraorder Caridea Superfamily Atyoidea Family Atyidae Genus Caridina Species Caridina propinqua De Man, 1908 – bengal caridina” “Taxonomic Status: Current Standing: valid” Size, Weight, and Age Range From Cai and Shokita (2006): “Material examined […] 11 males, cl [carapace length] 2.6–3.3 mm, 6 females, cl 3.5–3.8 mm, 16 ovigerous females, cl 3.4–4.0 mm […]” 2 Environment From Cai and Shokita (2006): “Lower reaches of rivers or mountain streams which discharge to the sea. -

Weddell Design Forum a Summary of the Outcomes of a Workshop to Explore Issues + Options for the Future City of Weddell
Weddell Design Forum A Summary of the Outcomes of a Workshop to explore Issues + Options for the future City of Weddell 27 September - 1 October 2010 Darwin Convention Centre, Darwin A report to the Northern Territory Government from the consultant leaders of the Weddell Design Forum NOVEMBER 2010 Sustainable, Liveable Tropical, Contents Foreword By 2018, Palmerston will be at capacity, vacant blocks around Introduction 1 Darwin will be developed. Darwin is one of the fastest growing cities in Australia. While much of this growth will be absorbed by The Site + Growth Context 2 in-fill development in Darwin’s existing suburbs and new Palmerston suburbs, we have to start planning now for the future growth of the Community Input 4 Territory. Special Interest Groups 5 Our children and grandchildren will want to live in houses that are in-tune with our environment. They will want to live in a community Key Issues for Weddell 6 that connects people with schools, work, shops and recreation Scenarios 9 facilities that are within walking distance rather than a car ride away. Design Outcomes 10 Achieving this means starting now with good land use planning, setting aside strategic transport corridors and considering the Traffic + Streets 26 physical and social services that will be needed. Indicative Development Costs 27 The Weddell Conference and Design Forum gave us a blank canvas Conclusions + Recommendations 28 on which to paint ideas, consider the picture and recalibrate what we had created. Weddell Design Team 30 It was a unique opportunity rarely experienced by our planning professionals and engineers to consider the views of the community, the imagination of our youth and to engage with such a range of experts. -

De Grave & Fransen. Carideorum Catalogus
De Grave & Fransen. Carideorum catalogus (Crustacea: Decapoda). Zool. Med. Leiden 85 (2011) 407 Fig. 48. Synalpheus hemphilli Coutière, 1909. Photo by Arthur Anker. Synalpheus iphinoe De Man, 1909a = Synalpheus Iphinoë De Man, 1909a: 116. [8°23'.5S 119°4'.6E, Sapeh-strait, 70 m; Madura-bay and other localities in the southern part of Molo-strait, 54-90 m; Banda-anchorage, 9-36 m; Rumah-ku- da-bay, Roma-island, 36 m] Synalpheus iocasta De Man, 1909a = Synalpheus Iocasta De Man, 1909a: 119. [Makassar and surroundings, up to 32 m; 0°58'.5N 122°42'.5E, west of Kwadang-bay-entrance, 72 m; Anchorage north of Salomakiëe (Damar) is- land, 45 m; 1°42'.5S 130°47'.5E, 32 m; 4°20'S 122°58'E, between islands of Wowoni and Buton, northern entrance of Buton-strait, 75-94 m; Banda-anchorage, 9-36 m; Anchorage off Pulu Jedan, east coast of Aru-islands (Pearl-banks), 13 m; 5°28'.2S 134°53'.9E, 57 m; 8°25'.2S 127°18'.4E, an- chorage between Nusa Besi and the N.E. point of Timor, 27-54 m; 8°39'.1 127°4'.4E, anchorage south coast of Timor, 34 m; Mid-channel in Solor-strait off Kampong Menanga, 113 m; 8°30'S 119°7'.5E, 73 m] Synalpheus irie MacDonald, Hultgren & Duffy, 2009: 25; Figs 11-16; Plate 3C-D. [fore-reef (near M1 chan- nel marker), 18°28.083'N 77°23.289'W, from canals of Auletta cf. sycinularia] Synalpheus jedanensis De Man, 1909a: 117. [Anchorage off Pulu Jedan, east coast of Aru-islands (Pearl- banks), 13 m] Synalpheus kensleyi (Ríos & Duffy, 2007) = Zuzalpheus kensleyi Ríos & Duffy, 2007: 41; Figs 18-22; Plate 3. -

Assessment of the Risk to Norwegian Biodiversity from Import and Keeping of Crustaceans in Freshwater Aquaria
VKM Report 2021: 02 Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria Scientific Opinion of the Panel on Alien Organisms and Trade in Endangered Species of the Norwegian Scientific Committee for Food and Environment VKM Report 2021: 02 Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria. Scientific Opinion of the Panel on Alien Organisms and trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food and Environment 15.02.2021 ISBN: 978-82-8259-356-4 ISSN: 2535-4019 Norwegian Scientific Committee for Food and Environment (VKM) Postboks 222 Skøyen 0213 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] vkm.no vkm.no/english Cover photo: Mohammed Anwarul Kabir Choudhury/Mostphotos.com Suggested citation: VKM, Gaute Velle, Lennart Edsman, Charlotte Evangelista, Stein Ivar Johnsen, Martin Malmstrøm, Trude Vrålstad, Hugo de Boer, Katrine Eldegard, Kjetil Hindar, Lars Robert Hole, Johanna Järnegren, Kyrre Kausrud, Inger Måren, Erlend B. Nilsen, Eli Rueness, Eva B. Thorstad and Anders Nielsen (2021). Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria. Scientific Opinion of the Panel on Alien Organisms and trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food and Environment. VKM report 2021:02, ISBN: 978-82-8259- 356-4, ISSN: 2535-4019. Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway. 2 Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria Preparation of the opinion The Norwegian Scientific Committee for Food and Environment (Vitenskapskomiteen for mat og miljø, VKM) appointed a project group to draft the opinion. -

Lines of Evidence for the Validity of Caridina Buehleri Roux (Crustacea : Decapoda : Atyidae) and for C
When molecules and morphology work together: lines of evidence for the validity of Caridina buehleri Roux (Crustacea : Decapoda : Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym Valentin de Mazancourt, Gerard Marquet, Werner Klotz, Philippe Keith, Magalie Castelin To cite this version: Valentin de Mazancourt, Gerard Marquet, Werner Klotz, Philippe Keith, Magalie Castelin. When molecules and morphology work together: lines of evidence for the validity of Caridina buehleri Roux (Crustacea : Decapoda : Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym. Invertebrate Systematics, CSIRO Publishing, 2017, 31 (2), pp.220. 10.1071/IS16044. hal-02171153 HAL Id: hal-02171153 https://hal.archives-ouvertes.fr/hal-02171153 Submitted on 2 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 When molecules and morphology work together: lines of evidence for the validity of 2 Caridina buehleri Roux, 1934 (Crustacea: Decapoda: Atyidae) and for C. gueryi Marquet, 3 Keith & Kalfatak, 2009 as its junior synonym. 4 VALENTIN de MAZANCOURT1*, GERARD MARQUET1, WERNER KLOTZ3, PHILIPPE 5 KEITH1 AND MAGALIE CASTELIN1-2 6 7 1 Muséum national d’Histoire naturelle, DMPA, UMR 7208, CP026, 57, rue Cuvier, F-75231 8 Paris, Cedex 05 9 2 Aquatic Animal Health Section, Fisheries and Oceans Canada, Pacific Biological Station, 10 3190 Hammond Bay Road, Nanaimo, BC, Canada V9T 6N7 11 3 Wiesenweg 1, A-6063 Rum, Austria. -
![[PDF] Freshwater Crustacea of the Mimika Region, New Guinea](https://docslib.b-cdn.net/cover/9537/pdf-freshwater-crustacea-of-the-mimika-region-new-guinea-1739537.webp)
[PDF] Freshwater Crustacea of the Mimika Region, New Guinea
BioAccess Australia Short, J. W., 2009. Freshwater Crustacea of the Mimika region – New Guinea. PT Freeport Indonesia: Kuala Kencana, ix + 96 pp. Other publications by John W. Short BioAccess Australia biodiversity consultancy and publishing Freshwater Crustacea of the Mimika Region New Guinea John W. Short Published by: PT Freeport Indonesia Enviromental Department Jl. Mandala Raya Selatan No. 1, Kuala Kencana, Timika 99920 Papua Design: John W. Short Cover Design: Hilman Ansori Printed by: PT. Indonesia Printer © John W. Short, 2009. All material in this book is copyright and may not be reproduced except with the written permission of the publishers. ISBN 978-979-97593-3-4 Cover illustration: Southern New Guinean River Prawn, Macrobrachium sp. 1 (Photo by John W. Short) Title Page Illustrations: Spiny Pacific Fan Shrimp, Atyopsis spinipes (after Cowles, 1914), top; Knob- fingered River Prawn, Macrobrachium mammillodactylus (Photo by John W. Short), bottom. Foreword As in my previous foreword for the Mangrove Estuary Crabs of the Mimika Region book, I herewith would like to thank Dr. John W. Short and all contributors in the Freshwater Crustacea of the Mimika Region book for their hard work and dedication towards documenting the freshwater crustacean habitat in the region for the benefit of broader society. This is the seventh biodiversity book published by PT Freeport Indonesia, in its quest to continuously disseminate knowledge related to biodiversity to the public. PT Freeport Indonesia, in more than 40 years of its operations in Papua, finds itself located in one of the most exotic and unique environments in the world. The island is one of the most biologically diverse mangrove estuary ecostystems and its 1.75 million hectares of mangrove vegetation is the largest in Indonesia. -

Flood Watch Areas Arnhem Coastal Rivers Northern Territory River Basin No
Flood Watch Areas Arnhem Coastal Rivers Northern Territory River Basin No. Blyth River 15 Buckingham River 17 East Alligator River 12 Goomadeer River 13 A r a f u r a S e a Goyder River 16 North West Coastal Rivers Liverpool River 14 T i m o r S e a River Basin No. Adelaide River 4 below Adelaide River Town Arnhem Croker Coastal Daly River above Douglas River 10 Melville Island Rivers Finniss River 2 Island Marchinbar Katherine River 11 Milikapiti ! Island Lower Daly River 9 1 Elcho ! Carpentaria Coastal Rivers Mary River 5 1 Island Bathurst Nguiu Maningrida Galiwinku River Basin No. Island 12 ! ! Moyle River 8 ! Nhulunbuy 13 Milingimbi ! Yirrkala ! Calvert River 31 South Alligator River 7 DARWIN ! ! Howard " Oenpelli Ramingining Groote Eylandt 23 Tiwi Islands 1 2 Island 17 North West 6 ! 14 Koolatong River 21 Jabiru Upper Adelaide River 3 Coastal 15 Batchelor 4 Limmen Bight River 27 Wildman River 6 Rivers ! 16 7 21 McArthur River 29 3 5 ! Bickerton Robinson River 30 Island Daly River ! Groote Roper River 25 ! ! Bonaparte Coastal Rivers Bonaparte 22 Alyangula Eylandt Rosie River 28 Pine 11 ! 9 Creek Angurugu River Basin No. Coastal 8 Towns River 26 ! ! Kalumburu Rivers Numbulwar Fitzmaurice River 18 ! Walker River 22 Katherine 25 Upper Victoria River 20 24 Ngukurr 23 Waterhouse River 24 18 ! Victoria River below Kalkarindji 19 10 Carpentaria G u l f 26 Coastal Rivers ! o f ! Wyndham Vanderlin C a r p e n t a r i a ! 28 Kununurra West Island Island 27 ! Borroloola 41 Mount 19 Barnett Mornington ! ! Dunmarra Island Warmun 30 (Turkey 32 Creek) ! 29 Bentinck 39 Island Kalkarindji 31 ! Elliott ! ! Karumba ! 20 ! Normanton Doomadgee Burketown Fitzroy ! Crossing Renner ! Halls Creek ! Springs ! ! Lajamanu 41 Larrawa ! Warrego Barkly ! 40 33 Homestead QLD ! Roadhouse Tennant ! Balgo Creek WA ! Hill Camooweal ! 34 Mount Isa Cloncurry ! ! ! Flood Watch Area No. -

Flood Risk Management in Australia Building Flood Resilience in a Changing Climate
Flood Risk Management in Australia Building flood resilience in a changing climate December 2020 Flood Risk Management in Australia Building flood resilience in a changing climate Neil Dufty, Molino Stewart Pty Ltd Andrew Dyer, IAG Maryam Golnaraghi (lead investigator of the flood risk management report series and coordinating author), The Geneva Association Flood Risk Management in Australia 1 The Geneva Association The Geneva Association was created in 1973 and is the only global association of insurance companies; our members are insurance and reinsurance Chief Executive Officers (CEOs). Based on rigorous research conducted in collaboration with our members, academic institutions and multilateral organisations, our mission is to identify and investigate key trends that are likely to shape or impact the insurance industry in the future, highlighting what is at stake for the industry; develop recommendations for the industry and for policymakers; provide a platform to our members, policymakers, academics, multilateral and non-governmental organisations to discuss these trends and recommendations; reach out to global opinion leaders and influential organisations to highlight the positive contributions of insurance to better understanding risks and to building resilient and prosperous economies and societies, and thus a more sustainable world. The Geneva Association—International Association for the Study of Insurance Economics Talstrasse 70, CH-8001 Zurich Email: [email protected] | Tel: +41 44 200 49 00 | Fax: +41 44 200 49 99 Photo credits: Cover page—Markus Gebauer / Shutterstock.com December 2020 Flood Risk Management in Australia © The Geneva Association Published by The Geneva Association—International Association for the Study of Insurance Economics, Zurich. 2 www.genevaassociation.org Contents 1. -

A Compendium of Ecological Information on Australia's Northern
A Compendium of Ecological Information on Australia’s Northern Tropical Rivers REPORT 7 Freshwater Fish Damien BurrowsA AAustralian Centre for Tropical Freshwater Research, James Cook University, Townsville Queensland 4811 Australia Authors This report should be cited as follows: Burrows, D. 2008. In G.P. Lukacs and C.M. Finlayson (eds) 2008. A Compendium of Ecological Information on Australia’s Northern Tropical Rivers. Sub-project 1 of Australia’s Tropical Rivers – an integrated data assessment and analysis (DET18). A report to Land & Water Australia. National Centre for Tropical Wetland Research, Townsville, Queensland. Contact information NCTWR C/ Australian Centre for Tropical Freshwater Research James Cook University Townsville 4811 Queensland Australia Funding statement This project was funded by the Natural Heritage Trust Phase 2 (NHT2) and Land & Water Australia (LWA) as part of the Tropical Rivers Inventory and Assessment Project (TRIAP). Disclaimer The views and opinions expressed in this report do not necessarily reflect those of the National Centre for Tropical Wetlands Research and its partners. While reasonable efforts have been made to ensure that the contents of this report are factually correct, some essential data rely on the references cited and the NCTWR do not accept responsibility for the accuracy, currency or completeness of the contents of this report, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the report. Readers should exercise