São Paulo 2018 Plan to Attend 2018 Latin American Mds Foundation Symposium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High Schools of Undergraduates and New Freshmen Fall 2007
IR Report Series Vol. 078, No. 007 November 2, 2007 High Schools of Undergraduates and New Freshmen Fall 2007 This report contains a list of high schools attended by undergraduates who were enrolled in the fall 2007 term. Section I contains data for all undergraduates and Section II contains data for new freshmen only. The schools are sorted in descending order by the number of undergraduates (or new freshmen from that high school). Office of Institutional Research and Effectiveness 7502 Fondren Road • Houston, Texas 77074-3298 • 281-649-3466 Section I: High Schools of Undergraduates, Fall 2007 2 High School* # % I H Kempner HS Sugar Land Texas 60 4.5 William P Clements HS Sugar Land Texas 59 4.4 Home Schooled Houston Texas 52 3.9 Stephen F Austin HS Sugar Land Texas 37 2.8 John Foster Dulles HS Sugar Land Texas 35 2.6 Elkins HS Missouri City Texas 30 2.2 Elsik HS Houston Texas 28 2.1 Bellaire Senior HS Bellaire Texas 27 2.0 Kerr HS Houston Texas 26 1.9 Alief Hasting High School Houston Texas 25 1.9 Westside HS Houston Texas 24 1.8 L V Hightower HS Missouri City Texas 22 1.6 Cinco Ranch HS Katy Texas 19 1.4 James E Taylor HS Katy Texas 17 1.3 Stephen F Austin Sr HS Houston Texas 16 1.2 George Bush High School Richmond Texas 15 1.1 Jersey Village HS Houston Texas 14 1.0 Pearland HS Pearland Texas 14 1.0 Cy-Fair Senior HS Cypress Texas 13 1.0 Mayde Creek HS Houston Texas 13 1.0 Stafford HS Stafford Texas 13 1.0 Debakey HS Health Professions Houston Texas 12 0.9 Fort Bend Baptist Academy Sugarland Texas 12 0.9 Alief Taylor High School Houston Texas 11 0.8 Cypress Ridge High School Houston Texas 11 0.8 Humble HS Humble Texas 11 0.8 Mirabeau B Lamar Sr HS Houston Texas 11 0.8 North Shore Senior HS Houston Texas 11 0.8 Cypress Falls HS Houston Texas 10 0.7 Foster High School Richmond Texas 10 0.7 Klein Oak HS Spring Texas 10 0.7 S P Waltrip Senior HS Houston Texas 10 0.7 Spring HS Spring Texas 10 0.7 Spring Woods Sr HS Houston Texas 10 0.7 (Continued) *High School data may not be available for each student in the class. -
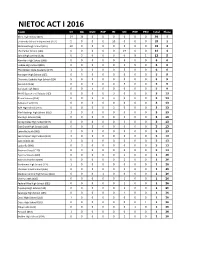
Nietoc Act I 2016
NIETOC ACT I 2016 Team DI DA DUO EXP HI OO POE PRO Total Place Akins High School (001) 14 16 0 0 0 0 0 0 30 1 University School Independent (152) 5 0 0 0 20 0 0 0 25 2 McDowell High School (097) 20 0 0 0 0 0 0 0 20 3 The Harker School (148) 0 0 0 0 0 14 0 0 14 4 Salina High Central (134) 0 3 0 0 0 0 9 1 13 5 Hamilton High School (066) 0 0 0 0 0 6 0 0 6 6 Lindale High School (089) 0 0 0 0 0 1 5 0 6 6 The Golden State Academy (147) 1 0 0 0 0 5 0 0 6 6 Harlingen High School (067) 0 5 0 0 0 0 0 0 5 9 Charlotte Catholic High School (024) 5 0 0 0 0 0 0 0 5 9 Scarsdale (136) 0 0 0 0 0 5 0 0 5 9 Carlsbad High (016) 0 0 5 0 0 0 0 0 5 9 MLHS Speech and Debate (103) 0 0 0 0 3 0 0 0 3 13 Bronx Science (014) 0 0 0 0 3 0 0 0 3 13 Bellevue East (011) 0 0 3 0 0 0 0 0 3 13 Ryle High School (131) 0 0 0 0 3 0 0 0 3 13 Alief Hastings High School (002) 3 0 0 0 0 0 0 0 3 13 Van High School (154) 0 0 0 0 0 3 0 0 3 13 Spring Valley High School (144) 0 0 0 0 0 3 0 0 3 13 Oak Grove High School (115) 0 0 3 0 0 0 0 0 3 13 Lakeville South (082) 3 0 0 0 0 0 0 0 3 13 Sacred Heart High School (132) 3 0 0 0 0 0 0 0 3 13 Oak Knoll (116) 3 0 0 0 0 0 0 0 3 13 Louisville (090) 0 3 0 0 0 0 0 0 3 13 Naaman Forest (110) 0 3 0 0 0 0 0 0 3 13 Cypress Woods (039) 0 0 0 0 0 1 0 0 1 26 BASIS Indepedent (009) 0 0 0 0 0 1 0 0 1 26 Northwest High School (114) 1 0 0 0 0 0 0 0 1 26 The Bear Creek School (146) 0 0 0 0 0 1 0 0 1 26 Madison Central High School (092) 0 0 0 1 0 0 0 0 1 26 Cherry Creek (026) 0 0 0 0 0 1 0 0 1 26 Federal Way High School (052) 0 0 0 0 0 1 0 0 1 26 Puyallup High -

In Puerto Rico, Michigan Reducing the Number of Credits and of Engineering, Respectively Agr
CoHemis...update Overcoming through cooperation Founded with the support of the National Science Foundation (NSF) December 1999 University of Puerto Rico at Mayagüez (UPRM) Vol. 9, No.1 CoHemis facilitates COHEMIS DELIVERS NEW COLLABORATIONS AND course in Uruguay RENEWS OLD CONTACTS IN CENTRAL AMERICA Nitrogen Fixation Module In the Office of the On July, 1999, the Foundation for Chancellor of Microbiology approved an $18,000 grant Guatemala’s for a Nitrogen Fixation Education Mod- University of San ule for Uruguay. The two-year proposal Carlos, CoHemis’ was submitted by Dr. Salvador Curbelo, codirectors Dr. Jorge Vélez-Arocho and Dr. a professor at Uruguay’s Universidad Luis Pumarada- La República, a CoHemis Consortium O’Neill receive USC member. The course’s main resource pins from Chancellor will be Dr. Eduardo Schroder, from the Efrain Medina and UPRM Faculty of Agricultural Sciences. Dean of Engineering Herbeth Miranda- As required by the Foundation, CoHe- Barrios. San Carlos mis will administer the funds as a free was founded in 1676 contribution to the project. by a decree of The 4-week course will be taught to Spain’s Charles II. undergraduate students the first year, and to graduate students in the second. It will alternate theory and laboratory ex- The co-directors of the CoHemis Rico Water Resources and Environmen- periences with visits to the innoculant Center, Drs. Luis Pumarada-O’Neill tal Research Institute, traveled to Central industry and agriculture research cen- and Jorge I. Vélez-Arocho, along with America in mid July, 1999. Drs. Puma- ters. The project will invite other ex- Dr. -
Houston's Learning Curve
Inside Outlook: Whichbattle defines Texas history? 16B Houston Chronicle | houstonchronicle.com and chron.com | Sunday, April 21, 2013 | Section B xxx SCHOOL REPORTCARD SPECIAL COVERAGEPAGES B2-9 Houston’slearning curve HISD has the most at both top,bottom of rankings; magnets and charters fare well By Ericka Mellon In her sixth-grade historyclass at asmall school in the Montrose area, 11-year-old Patrice Stubblefield readquietlyfrom her textbook: “Subió el precio delpetróleo.” She turned to twoclassmates at her table and explained in Englishthatthe price of petro- leum rose in Latin America in 1980. “It’sAmérica Latina,” corrected Gresia Nunez, 12,the daughter of Mexican immi- grants. Nunez learned to speak and readEnglish as ayoung studentatWharton Dual Lan- guage Academy,while Stubblefield learned Spanishatthe school. At Wharton, native Englishspeakers and native Spanishspeak- ers studysidebyside, immersed in Spanish in the early gradeswithmore and more Englishintegrated as they getolder. Theformula has worked well for Wharton, aHouston IndependentSchool District campus serving students in pre- kindergarten througheighthgrade.The middle school levelearned an “A”grade this year from Children at Risk, alocal research and advocacy nonprofit thatannuallyranks public schools across Texas. Theelemen- taryschool earned a“B.” Roughlyaquarter of the schools in Texas earning A’s, based on their academics and other classroom factors, are in the eight- county greater Houston area, according to the Children at Riskanalysis released to the Houston Chronicle. Houston ISD dominated the top and the bottom of the local rankings. On the high school list, DeBakey High School for Health MelissaPhillip /HoustonChronicle Professions in HISD ranked firstlocally Gresia Nunez, 12, from left,Brianna Ward, 12, and Patrice Stubblefield, 11,workintheir sixth-grade geography class and third in the state. -

National Blue Ribbon Schools Recognized 1982-2015
NATIONAL BLUE RIBBON SCHOOLS PROGRAM Schools Recognized 1982 Through 2015 School Name City Year ALABAMA Academy for Academics and Arts Huntsville 87-88 Anna F. Booth Elementary School Irvington 2010 Auburn Early Education Center Auburn 98-99 Barkley Bridge Elementary School Hartselle 2011 Bear Exploration Center for Mathematics, Science Montgomery 2015 and Technology School Beverlye Magnet School Dothan 2014 Bob Jones High School Madison 92-93 Brewbaker Technology Magnet High School Montgomery 2009 Brookwood Forest Elementary School Birmingham 98-99 Buckhorn High School New Market 01-02 Bush Middle School Birmingham 83-84 C.F. Vigor High School Prichard 83-84 Cahaba Heights Community School Birmingham 85-86 Calcedeaver Elementary School Mount Vernon 2006 Cherokee Bend Elementary School Mountain Brook 2009 Clark-Shaw Magnet School Mobile 2015 Corpus Christi School Mobile 89-90 Crestline Elementary School Mountain Brook 01-02, 2015 Daphne High School Daphne 2012 Demopolis High School Demopolis 2008 East Highland Middle School Sylacauga 84-85 Edgewood Elementary School Homewood 91-92 Elvin Hill Elementary School Columbiana 87-88 Enterprise High School Enterprise 83-84 EPIC Elementary School Birmingham 93-94 Eura Brown Elementary School Gadsden 91-92 Forest Avenue Academic Magnet Elementary School Montgomery 2007 Forest Hills School Florence 2012 Fruithurst Elementary School Fruithurst 2010 George Hall Elementary School Mobile 96-97 George Hall Elementary School Mobile 2008 1 of 216 School Name City Year Grantswood Community School Irondale 91-92 Guntersville Elementary School Guntersville 98-99 Heard Magnet School Dothan 2014 Hewitt-Trussville High School Trussville 92-93 Holtville High School Deatsville 2013 Holy Spirit Regional Catholic School Huntsville 2013 Homewood High School Homewood 83-84 Homewood Middle School Homewood 83-84, 96-97 Indian Valley Elementary School Sylacauga 89-90 Inverness Elementary School Birmingham 96-97 Ira F. -

Electrical Service Order Form Located on Pages 65-66 of the Event Planning Guide
Your Northeast Business Address OneOne Convention Miss America Boulevard Way AtlanticAtlantic City,City, NJ 0840108401 Phone:609/449-2000 609-449-2000 Fax:Fax 609-449-2090609/449-2090 www.accenter.com Welcome to the Atlantic City Convention Center, America’s Northeast Business Address! We have prepared this Event Planning Guide to provide you with information to help make your event a success, whether you are booking a convention, trade show, meeting, consumer show, concert, dance, sporting event or any other kind of special activity. Our experienced staff will provide additional information and guidance throughout the planning stages, from your initial consultation to the successful culmination of your event. An Event Manager will be assigned to your event. We encourage you to communicate with your Event Manager directly and as often as necessary. Keeping the doors of communication open is a major step toward ensuring the success of your show. We are proud you have chosen the Atlantic City Convention Center and look forward to working with you and your staff. Sincerely, Charles F. Beirne, Regional General Manager Atlantic City Convention Center/SMG Atlantic City Convention Center TABLE OF CONTENTS I. Introduction 3-4 Frequently Asked Questions 5-6 II. Location Maps/Directions/Transportation 7-10 III. Insurance 11-12 IV. Event Services/Support Services 13-21 Event Manager 13 Public Safety 13-14 Security & Police 14 Electrical/Utilities 15 Atlantic City Convention Center Utility Services for Events 15 Parking 15 Event Services Estimate 15 Presentation Services Audio Video (PSAV) 16 Marketing & Media Services 17-18 Advanced Technology at the Atlantic City Convention Center 19 Meeting Planners Check List & Timeline 20-21 V. -

Alief Independent School District 2020-21 Official Budget
Alief Independent School District 2020-21 Official Budget Alief Independent School District 4250 Cook Road Houston, Texas 77072 www.aliefisd.net Alief Independent School District Houston, Texas 2020-21 Official Budget Effective September 1, 2020 – August 31, 2021 Issued by: H.D. Chambers Superintendent Administrative Services Division Charles Woods Deputy Superintendent for Business Deanna Wentz, CPA Assistant Superintendent of Finance Table of Contents 2020-21 Budget Introductory Section Principal Officials .............................................................................................................................. 1 Executive Summary .......................................................................................................................... 2 School Board of Trustees ............................................................................................................... 10 District-wide Organizational Chart .................................................................................................. 12 Enrollment by Campus ................................................................................................................... 13 District Map ..................................................................................................................................... 14 Classification of Revenues and Expenditures ................................................................................ 15 Combined Budget Summaries ....................................................................................................... -

Duet Acting 2013 TFA State Tournament
Duet Acting 2013 TFA State Tournament Code Name School Prelims Cume Qtr Cume Semi Cume Final Cume Mariah Rivas CK Amanda Taylor Abilene Cooper High School 6 3 3 12 Sidney Odom XG Abigail Onwunali Alief Hastings High School 2 2 5 9 1 1 1 3 4 5 4 13 Tam Tran DX Alex Vinh Alief Kerr High School 6 4 4 14 Jimmy Frazier FK Jeanae Jackson Angleton High School 6 4 6 16 Xavier Lonvelin PO Savanna Saldana Bel Air High School 5 6 6 17 Camille Acosta TA Cristian Apodaca Burges 6 6 6 18 Sarah Al-shalash DZ Zach Royal Centennial High School 2 2 4 8 4 1 3 8 5 2 5 12 Paige LaNasa DZ Olivia Genusa Centennial High School 3 6 5 14 JAMES ELKINS NN LAUREN STRICKLAND Central High School 4 2 3 9 6 6 4 16 CONNER MCLAUGHLIN NN ADDISON WALKER Central High School 6 3 5 14 COBY EVERS NN BRYAN BASS Central High School 4 1 2 7 5 2 2 9 1 6 1 8 3 6 4 2 5 20 Raul Boswell HN Laura Mendez-Oronoz Chapin High School 6 5 6 17 Courtney Jaekel NO Sarah Bennett Clear Creek High School 6 5 6 17 Katelynn Barba NO Jessica Leach Clear Creek High School 6 6 6 18 Blake Rushing DP Cristina Pop Creekview High School 2 1 2 5 1 2 1 4 2 3 4 9 Maddie Wright DP Michael Ferguson Creekview High School 5 1 3 9 6 6 6 18 Maria Lozano DP Natalie Walker Creekview High School 1 4 1 6 3 2 1 6 6 3 4 13 Terrica Bass TK Dallas Sanchez Cypress Lakes High School 5 6 5 16 Jarod King HZ Joanna Godinez Cypress Springs High School 4 3 4 11 Michelle Hoch OP Kate Loving Cypress Woods High School 6 6 1 13 Zachary McNeal OP Aidan Ferrer Cypress Woods High School 4 5 6 15 Sergio Bernal CD Cosme Flores Donna High -

Strategies for Success
HoustonChronicle @HoustonChron Houston Chronicle | Sunday, June 5, 2016 |HoustonChronicle.com and Chron.com Section N xx PRE-K COLLEGE PREP SCHOOL CHOICE A head start Giving guidance Opening doors KIPP Explore starts working Schools are adding counselors Financial aid can make even with 3- and 4-year-olds to erase to help navigate the tricky pricey private schools a disadvantages. Page N3 application process. Page N4 viable alternative. Page N7 SCHOOL REPORT CARD Strategies forsuccess By Ericka Mellon High-poverty schools earn true: SchoolS with greater concentrationS of low- income StudentS are more likely to rank lower. gold marks for programs The high-poverty campuSeS that riSe to the aulette CaSton, the principal toptypically are magnet, charter or Specialty of Ed White Elementary in that boost academics schools that require StudentS to apply. SouthweSt HouSton, knowS TheNo. 1-ranked SchoolS were DeBakeyHigh her StudentS enter School at a School for HealthProfeSSionS, a magnet school PdiSadvantage. Many are new to with admissionSStandards; T.H.Rogers, amag- the country and not uSed to attending daily, net school serving students in kindergarten Structured claSSeS. And more than eight out througheighthgrade whoqualifyasgifted;and of 10 come from low-income families. RiverOaksElementary, where 10 percent of the To catch up the StudentS, CaSton haS recruit- Students are lowincome. ed retired teacherS to Serve aS tutorS, kept a So- About aquarterofHouston ISD’s schoolS cial worker on Staff part time and promoted an earned “A”grades, and 30 percentreceivedan after-School homework club So the children can “F”, according to therankings, published today get help before leaving to care for SiblingS while in thiS Special Section of the HouStonChronicle. -

Houston 2012 Fellows
Houston 2012 Fellows This summer, 67 Houston teachers representing 50 schools embark on self- designed learning odysseys as scholars, researchers, adventurers and Fund for Teachers Fellows. After pursuing scientific data, participating in seminars, volunteering with community organizations and observing best practices, these teachers will return to their classrooms as lead learners to inspire their students and school communities. Elementary Guillermo Ovalles Angelia Seagroves Garrett Elementary Hubenak Elementary Kelly Caldwell, April Davis, Tawanna Evans, Participate in teacher training in Helsinki, Discover the origins of Grimm’s fairy tales Destiny Parker and Brooke Wilso Finland, followed by a cultural immersion along the Fairy Tale Road in Germany and Burton Elementary language program sponsored by the also explore the Dachau concentration Visit Underground Railroad and national University of Turku, to examine Finnish camp to create K-5 library lessons that reach landmarks in New York, Philadelphia and teaching methods and develop an younger and older students and encourage Washington DC to broaden knowledge of instructional model that prepares students connections between texts and their own the Black American experience and more of all backgrounds for academic success. experiences. seamlessly incorporate African-American history with general social studies. Nancy Hess Nicki Frankie Griffin Elementary Janowski Elementary Jean King Research proactive renewable Explore Alaska’s Glacier Bay National Park De Zavala Elementary environments in Iceland, Norway, Denmark and Preserve in order to enhance content Follow the lives and careers of Gaudí, Miró and Sweden to initiate student discussions knowledge and develop a more enriching & Dalí across the Catalonia region of Spain, about renewable energies and the transition earth science unit. -

JEA/NSPA Fall National High School Journalism Convention November 1-4, 2018 • Hyatt Regency Chicago
JEA/NSPA Fall National High School Journalism Convention November 1-4, 2018 • Hyatt Regency Chicago JEA/NSPA Fall 2018 • CHICAGO — 1 PARK SCHOLAR PROGRAM A once-in-a-lifetime opportunity awaits outstanding high school seniors. A full scholarship for at least 10 exceptional communications students that covers the four-year cost of attendance at Ithaca College. Take a chance. Seize an opportunity. Change your life. Study at one of the most prestigious communications schools in the country—Ithaca College’s Roy H. Park School of Communications. Join a group of bright, competitive, and energetic students who are committed to using mass communication to make a positive impact on the world. To apply for this remarkable opportunity and to learn more, contact the Park Scholar Program director at [email protected] or 607-274-3089. ithaca.edu/parkscholars 2 — JEA/NSPA Fall 2018 • CHICAGO Twitter: @nhsjc/#nhsjc PARK SCHOLAR CONTENTS 4 Convention Officials PROGRAM 5 Local Team/One Story A once-in-a-lifetime opportunity awaits 6 Convention Rules/App outstanding high school seniors. 7 Keynote Speaker A full scholarship for at least 10 exceptional communications 8 Special Activities students that covers the four-year cost of attendance at Ithaca College. 10 Featured Speakers 14 Exhibitors/Advertisers 15 Sponsors 18 JEA Awards 20 NSPA Awards 25 Thursday at a Glance 25 Thursday Sessions 32 Friday at a Glance 39 Write-off Rooms 40 Friday Sessions 68 Saturday at a Glance 75 Saturday Sessions Take a chance. 98 Speaker Bios Seize an opportunity. 130 Hotel Floor Plans Change your life. Study at one of the most prestigious communications schools in the country—Ithaca College’s Roy H. -

Pioneer Progress
The Magazine For Alumni & Friends Of Glenville State College Waco Center Nears Completion WV Veterans’ Legacy Project Book, Play, & Documentary Produced GSC Graduates Honored At Alumni Banquet Hidden Promise Program Continues Fall To Expand 2013 From Pete and Betsy Greetings to our Alumni and Supporters Betsy and I are extremely pleased to be writing another letter to you as we share our second issue of Pioneer Progress. Our inaugural issue in the fall of 2012 resulted in an abundance of verbal and written applause from many members of our extended Pioneer family. Therefore, we are proud to bring the fall 2013 issue to you as we showcase what our school has become because of people like you. This magazine contains snapshots and stories of our many successes that continue to take place on our campus and in the lives of our alumni and friends of Glenville State College. As you read the stories and photo captions, we hope you enjoy updates on news that we first brought to you a year ago. The beautiful Waco Center is taking shape on Mineral Road and will be completed by the first of the year to serve the next chapters of our athletic program and a multitude of services that we provide to our community, the region, and the state. We have included updates about our many successful initiatives including the Hidden Promise Scholars Program and the West Virginia Veterans’ Legacy Project. We can’t help the pride we feel as we read about the many positive and inspirational stories showcasing our generous, hardworking, and talented faculty, staff, students, alumni, and donors.