Mixing of Tikitere Geothermal Discharges with Sewage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
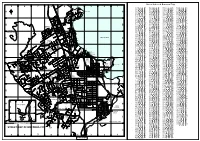
Street Index
PARAWAI RD Street Index of Rotorua City 9 8 7 6 5 4 3 2 1 MANAHI Adam Pl E-7 Gibson St H-2 Lytton St H-3 Ruby Pl E-8 AVE LIBRA Alison St E-7 Gifford Pl F-5 McCahon Dr F-8 Ruihi St G-3 PL Allan St G-4 Gillam Cres I-6 McCloskey Rd E-6 Russell Cres D-7 TUPARACRES GEMINI PL NAERA Amber Pl F-8 Gilltrap St E-5 McDowell St I-5 Russell Rd C-5 VISTA A ARIES PL A Amethyst Pl E-8 Goldie St F-8 McIntyre Ave H-2 Ruth St F-5 PL PL NORTH Amies Rd D-6 Goodwin Ave J-4 McKee Ave I-2 Rutland St G-5 LEO CAPRICORNPL PL Amohau Pl F-3 Gordon Rd D-7 McLean St H-3 Sala St I-2 GRAND TAURUSPL PAH RD VUE RD Amohau St F-3 Grand Vue Rd A-4 Mahana Pl I-5 Salisbury Rd C-5 AQUARIUS DR GRAND VUE RD Amohau St Extension F-3 Grayson Ave D-6 Mahanga Rd D-5 Sapphire Pl E-8 URQUHARTPL RIKA PL DARROCH Grey St G-3 Mahoe St H-2 Kawaha Amohia St F-3 G-4 Scott St BARNARD RD ST Primary KAWAHA POINT RD Amokura St B-6 Gwendoline St H-3 Maida Vale St H-2 Seddon St G-3 School Amun Pl H-6 Hamiora Pl G-1 Maisey Pl C-6 Sherriff St G-5 VIRGO AMOKURA PL TIRITAST Ann St G-4 Hamuera St C-4 Makitauna St D-3 Shirley St E-5 CHAPMAN JOHN LUKE PL ST RD Apollo Pl G-7 Hapi St H-6 Malfroy Rd G-4 Simmonds Cres I-5 PL Aquarius Dr A-5 Haratua Pl J-6 Mallard Dr B-6 Sloane Ave K-4 * KINGDOM KAWAHA POINT RD Arataua St D-3 Harold Cres F-5 Manahi Ave A-4 Solly Pl I-6 B ROWI ST KOUTU B LOGAN MATTHEW PL DR RD Arawa St E-3 Hathor St H-6 Manuka Cres H-4 Sophia St H-3 SELWYN ASHMORE ST PL Argus St G-6 Hatupatu Dr E-1 Marae St C-4 Spencer St E-8 HEIGHTS BELLVUE FENRUSS Ariariterangi St D-4 Houkotuku St D-3 Marcasite -

Part 2 Geological History.Indd
2. Geological History Activities Curriculum Curriculum Environmental Page Activity title level link education aspect 2a Formation of the L 3–5 Science 37 Rotorua lakes 2b Silent Card Shuffl e L 3–5 Science 39 2c Mt Ngongotaha L 3–5 Science About, In 42 2d Looking at Sediment Any level Science About, In 53 2e How Wet is your Any level Science 55 Sediment? 2f What is in your L 5 Science 58 Sediment? Relevant resources: • Rotorua’s Volcanic Past – video – hire from Rotorua Public Library • Rotorua Museum Education Resources » Te Arawa – Mai Maketu ki Tongariro » Legends, Landforms and Learning » Tarawera photo kit » Tarawera Resource kit • Pollution Busters newsletter # 5 Volcanoes • Learning Media - Building Science Concepts • Book 12 – Volcanoes: Hot Rock in a Cool World Levels: 3–4 • Book 52 – The Land Changes: Keeping Earth’s Systems in Balance: Levels: 3–4 • Learning Media – Readers » The Changing Land, Jane Buxton, ISBN 0478214162, explores how the elements can affect Earth and change its shape The Rotorua Lakes Education Resource 35 Activity 2a Formation of the Rotorua Lakes Curriculum links Science Any level 2 Resources required Science • PowerPoint presentation by Will Esler, University of Waikato (on CD) • The Life and Times of Lake Rotorua and Lake Rotoiti (see background notes) • Graphic of Rotorua lake edge 9000 years ago Method 1 View the PowerPoint (ppt) before using it with the class, or set a small group this responsibility. Identify good stopping places for discussion or questions. Have some questions already identifi ed e.g. What was the signifi cant event during this time? Why was it signifi cant? Who/what was responsible for this event occurring? If a small group has previewed the ppt then they can act as a lead questioner/facilitator for one of the groups below. -

Eight Existing Poverty Initiatives in NZ and the UK: a Compilation
Title page July 2017 Working Paper 2017/04 Eight Existing Poverty Initiatives in NZ and the UK: A compilation Working Paper 2017/04 Fact Sheets on Existing Initiatives: A compliation July 2017 Title Working Paper 2017/04 – Eight Existing Poverty Initiatives in NZ and the UK: A compilation Published Copyright © McGuinness Institute, July 2017 ISBN 978-1-98-851842-8 (Paperback) ISBN 978-1-98-851843-5 (PDF) This document is available at www.mcguinnessinstitute.org and may be reproduced or cited provided the source is acknowledged. Prepared by The McGuinness Institute, as part of the TacklingPovertyNZ project. Authors Alexander Jones and Ali Bunge Research team Ella Reilly and Eleanor Merton For further information McGuinness Institute Phone (04) 499 8888 Level 2, 5 Cable Street PO Box 24222 Wellington 6142 New Zealand www.mcguinnessinstitute.org Disclaimer The McGuinness Institute has taken reasonable care in collecting and presenting the information provided in this publication. However, the Institute makes no representation or endorsement that this resource will be relevant or appropriate for its readers’ purposes and does not guarantee the accuracy of the information at any particular time for any particular purpose. The Institute is not liable for any adverse consequences, whether they be direct or indirect, arising from reliance on the content of this publication. Where this publication contains links to any website or other source, such links are provided solely for information purposes and the Institute is not liable for the content of any such website or other source. Publishing This publication has been produced by companies applying sustainable practices within their businesses. -

Spas and Geothermal Fields Candice Chalmers Brock
ROTORUA ABSTRACT 1 Running head: ROTORUA ABSTRACT Rotorua Abstract Rotorua – Spas and Geothermal Fields Candice Chalmers Brock University Dave Brown Due: February 18 2010 TREN 3F94 ROTORUA ABSTRACT 2 In New Zealand’s North Island, a unique and popular tourist attraction can be found. Rotorua, a district that is filled with “...steaming hot springs, explosive geysers [and] bubbling mud pools...” (Lonely Planet, 2008, p.32) reminds tourists that they are in a destination that is incomparable. The harsh scent of sulphur fills the region, but this harmless element has been created by the geothermal activity that occurs in Rotorua (Bell, 1999). With ‘lunar landscapes’ that have been created by volcanic action and fourteen lakes, Rotorua attracts tourists of all kinds as this destination boasts a distinct natural environment (Ryan & Pike, 2003, p.315). The creation of these natural wonders have been because of Geothermal fields which have been produced by the ingredients of water, heat and access to the earth’s surface for geothermal heat. Once cold water moves through the ground towards the heat, the water naturally warms and moves upwards thus establishing geysers, fumaroles, mud pools and also hot springs (Department of Conservation). There are five main areas within Rotorua that possess these remarkable sights, and they are known as Whakarewarewa, Tikitere, Waimangu, Waiotapu and Orakei Korako (New Zealand on the Web). All are located in fairly close proximity, making them extremely accessible to visitors. With result many spas have been established throughout Rotorua, as there is undoubtedly an appeal to tourists as many are looking to achieve a personal experience with the natural products. -

Lake Rotoiti Ratepayers & Residents Association
Lake Rotoiti Ratepayers & Residents Association NEWSLETTER - SEPTEMBER 1995 Kia Ora from the Lake There have been signs lately that the many pleas of 'Enough is Enough' have been heeded. We are referring of course to the inordinate amount of rainfall experienced throughout June and July. Statistical evidence reveals that it rained every day except one from the middle of June to the latter part of July. According to long standing resident weather watcher, John Wright, rainfall to the end of July had almost equalled the total for the whole of 1994. Lake levels are the highest seen in recent times. We have also had some extremely cold spells with an easily discernible blanket of snow on Mt Ngongotaha and surrounding hills several weeks back. Notwithstanding this soggy saga, our vigil over matters of concern to local residents has continued to keep us busy. Appropriately, water is a central theme in most of the more prominent issues. In response to numerous requests, we are making renewed attempts to establish the level of support for a public water supply for West Rotoiti following the reticence of many to last years unsuccessful survey. Management of the Rotorua lakes both from an environmental and safety aspect has come under closer scrutiny this year with several Lakes Ratepayer Groups including our own calling for more expedient action to address obvious shortcomings. Rotorua District Council have now recognised the importance of the lakes to the extent of conceding to our calls to incorporate a stand alone chapter in the Proposed District Plan. An important event this year is the October elections to select our District and Regional Council representatives for the ensuing three year term. -

Mineral, Coal and Petroleum Resources: Production,Exploration and Potential
2.3 MINERAL, COAL AND PETROLEUM RESOURCES MINERAL, COAL AND PETROLEUM RESOURCES: PRODUCTION, EXPLORATION AND POTENTIAL Anthony B. Christie1, Richard G. Barker2 1 GNS Science, PO Box 30-368, Lower Hutt 5040, New Zealand 2 Consulting Geologist, PO Box 54-094, The Marina, Bucklands Beach, Auckland 2144, New Zealand ABSTRACT: New Zealand has been a signifi cant producer of minerals and coal since early European settlement in the mid-19th century, and of hydrocarbons since 1970. Current production consists of oil, gold and silver, high quality (bituminous) coal, ironsand and specialised industrial minerals such as halloysite china clay for export, and a range of minerals and rocks for domestic use that are fundamental to New Zealand’s economy and infrastructure. The latter include aggregate for road making and construction (concrete aggregate and cement), coal for use by industry and electricity generation, and limestone for agriculture, cement making, and industry. Small quantities of diatomite, dolomite, perlite, pumice, serpentinite, and zeolite are also produced mainly for domestic markets. Other commodities that have been produced in the past include antimony, chromium, copper, lead, manganese, mercury, phosphate, platinum, sulphur, tin, tungsten, and zinc. New Zealand has well-documented potential for the discovery of a wide range of minerals and of petroleum, both onshore and offshore. Exploration is essential for converting this resource potential to wealth-creating assets. The resource-related industries are signifi cant economic contributors, but New Zealand’s resource potential remains to be realised. Mineral resources are created by natural processes and are generally described as non-renewable, implying they are fi nite, but many minerals (metals in particular) have been produced for several thousand years. -

NZ) Publication #43 Page 1
The Bainbridge Church Story by J B Dawson 1983 Wesley Historical Society (NZ) Publication #43 Page 1 The Bainbridge Church Story by J B Dawson 1983 CONTENTS 1. Introductory. 2. Antecedents. 3. Beginnings, to 1906 4. Building, 1906 5. Struggle, 1907 to 1935. 6. Advance, 1936 to 1938. 7. War and Peace, 1939 to 1949 8. Jubilee, 1950 to 1954 9. Expansion, 1955 to 1964. 10. Tidying up', 1965 to 1978. 11. On the Move, 1979 to 1982. 12. Resurrection. Postscript. Ministers Acknowledgments Wesley Historical Society (NZ) Publication #43 Page 2 The Bainbridge Church Story by J B Dawson 1983 1. INTRODUCTORY On 16 December 1906 a Methodist church was opened and dedicated in Hinemoa Street, Rotorua. It was named after a young man who died in the Tarawera eruption. On 30 May 1982 the closing service of the church, now much enlarged, was held, over 300 people attending. The following day the demolition of the church, to make way for the erection of shops, was begun. Thus ended over 76 years of continual Methodist worship on this site. During that period the original membership of the church had been increased eleven fold, while the value of church and site had increased over a hundred fold. Rotorua Methodist Circuit retained a large supermarket building with offices, including the Church Office, overhead and an adjoining car park. These had been acquired and added to the original site. The following Sunday the congregation gathered for worship in borrowed school premises. Thus began its 'wilderness year' while the new Church Centre was being planned and erected on a site off Old Taupo Road. -

Rural/Central Athletics 2016 Tuesday, 22 November 2016 Rotorua International Stadium Field # 2
Rural/Central Athletics 2016 Tuesday, 22 November 2016 Rotorua International Stadium Field # 2 Official Results Highlighted names in yellow have qualified for the BOP Athletics Championships Track Results - 800 metres Place Open Boys - 800m School Time Record Record Holder 1st Oliver Larcombe Kaharoa 2.31.22 2.31.22 Oliver Larcombe – Kaharoa (2016) 2nd Austin Lash Otonga 2.40.75 3rd Ollie Weaver Kaharoa 2.43.04 4th Wiremu Pirika Ruamata 2.46.34 Place Open Girls – 800m School Time Record Record Holder 1st Annelyse Cowie Kaharoa 2.44.78 2.40.5 Hannah Gapes – Lynmore (2014) 2nd Poppy Martin St Marys 2.49.40 3rd Emma Eruni-Bennett Kaharoa 2.54.09 4th Angela Rowson Otonga 2.59.09 Track Results - 60 metres Place 9 Yr old Boys - 60m School Result Record Record Holder 1st Hamish Carr Otonga 8.90 8.66 Ariariterangi Tuhaka – Malfroy (2014) 2nd Tukotahi Richards-TeWhau Malfroy 9.03 3rd Caden Hughes Westbrook 9.22 4th Ezra Dorset Otonga 9.23 Place 9 Yr old Girls - 60m School Result Record Record Holder 1st Madison Landers Rotokawa 8.88 8.88 Madison Landers – Rotokawa (2016) 2nd Hanna Morrison Te Koutu 9.53 3rd Kate Shapley Glenholme 9.81 4th Ahipukahu Ruamata 9.83 Athletes with Disabilities – 100m Track Results Place Athletes with Disabilities Boys – 100m School Age Disability Result 1st Whitimai Waitoko Western Heights 9 AWDID 23.43 1st Whakapau Tumai-Te Pou Te Koutu 12+ AWDCP 20.11 Track Results – 100 metres Place 12+ Yr old Boys - 100m School Result Record Record Holder 1st Tikitere Patu Ruamata 13.60 13.60 Tikitere Patu – Ruamata (2016) 2nd Moana -

Evaluation of Potential Engineering Options for Reduction of Nitrogen Inputs to Lake Rotorua
EVALUATION OF POTENTIAL ENGINEERING OPTIONS FOR REDUCTION OF NITROGEN INPUTS TO LAKE ROTORUA R4181 EVALUATION OF POTENTIAL ENGINEERING OPTIONS FOR REDUCTION OF NITROGEN INPUTS TO LAKE ROTORUA Contract Report No. 4181 August 2017 Project Team: Jo McQueen-Watton - Report author William Shaw - Report author, peer review Prepared for: Toi Moana Bay of Plenty Regional Council P.O. Box 364 Whakatāne 99 SALA STREET, WHAKAREWAREWA, 3010, P.O. BOX 7137, TE NGAE, ROTORUA 3042 Ph 07-343-9017; Fax 07-343-9018, email [email protected], www.wildlands.co.nz CONTENTS 1. INTRODUCTION 1 2. OVERVIEW OF THE LAKE CATCHMENT 1 2.1 Ecological context 1 2.2 Geology 2 2.3 Lake Rotorua 2 2.4 Sub-catchments 4 3. KEY FACTORS AFFECTING NITROGEN REMOVAL 5 4. ASSESSMENT OF POTENTIAL TECHNOLOGIES 6 5. REJECTED TECHNOLOGIES 7 5.1 Overview 7 5.2 Nanobubbles 7 5.3 Flocculent application 8 5.4 Aeration-driven destratification 11 5.5 Lake bottom oxygenation 12 5.6 Wave barriers 13 5.7 Dredging 13 5.8 Grass carp 15 5.9 Hamurana diversion wall (with and without gates) 16 5.10 Wave barrier at Waiohewa 17 5.11 Floating wetlands 17 5.12 Removal of sewage from the catchment 19 6. POTENTIALLY FEASIBLE TECHNOLOGIES 20 6.1 Overview 20 6.2 Denitrification plant (same as Tikitere) 20 6.3 Lake weed harvesting 21 6.4 Natural wetlands: protection, maintenance and enhancement 22 6.5 Constructed wetlands 24 6.6 Denitrification beds/carbon walls 26 6.7 Watercress beds 28 6.8 Seepage wetlands and grass hedges 30 6.9 Anionic PAM blocks 32 6.10 Removal of N-fixing plant species from the catchment 33 6.11 Other considerations 35 7. -

Annual Report 2012-2013
ROTORUA TE ARAWA LAKES PROGRAMME Annual Report 2012-2013 Annual Programme Report 2012-2013 Table of Contents PURPOSE ......................................................................................................................................................... 4 CONTEXT.......................................................................................................................................................... 4 KEY ACHIEVEMENTS ......................................................................................................................................... 5 ROTORUA TE ARAWA LAKES ANNUAL WATER RESULTS ................................................................................... 6 LONG-TERM WATER QUALITY TRENDS ............................................................................................................ 7 LAKE ROTORUA................................................................................................................................................ 8 COMMENTS ON BEHIND SCHEDULE PROJECTS – ROTORUA ............................................................................................. 11 Reducing nutrients from rural land .............................................................................................................. 11 Tikitere ......................................................................................................................................................... 11 P-Locking ..................................................................................................................................................... -

ROTORUA CONSTITUENCY Ruato Rotoiti Lake Rotoma Lake Rotoitipaku Waiteti Bay Tikitere Tarukenga Ngongotaha Mamaku Lake Rotorua Te Ngae Tasman Mill Mokoia Is 0 0 0 0
Kotaro Kotaro Otanewainuku Te Ranga Otamamariri Stream Waiari Stream Pikowai Stream Te Ranga Waiwhakareto Stream Hukunui Stream Otanewainuku Whakahaupapa Stream Herepuru Stream Pongakawa Valley Pongakawa Valley 2775000 2780000 2785000 2790000 2795000 2800000 2805000 2810000 2815000 2820000 2825000 2830000 r Puwhenua e r v i e r R e v v r i i a R e Hiapo R a v w w i a a re R p o Pungarehu n WESTERN BAY OF PLENTY g a n a a Waipapa Stream p M a Stream Waipapa Stream m a n g Ngawaro u O CONSTITUENCY it n a a 0 K 0 0 M 0 0 0 5 5 5 Paretero 5 3 3 6 6 Hiwiroa gorewa River Man Te Pu Otuhepo Kaharoa 0 0 0 Tokerau 0 0 0 0 0 5 5 3 Manawahe 3 6 6 Okere Falls Otaramarae Karaponga Stream Kuhatahi Stream Whangamarino Oturoa Hamurana Ngarehu Point Lake Rotoehu EASTERN Waipare Stream Matutu Point Lake Rotoiti Rotoehu BAY OF PLENTY 0 Awahou Hinehopu 0 0 Mourea 0 0 Te Awahou Point M0 angaone Stream 5 CONSTITUENCY 5 4 Hauparu 4 3 Waimihia Bay Gisborne Point 3 Waihou River 6 6 Waiteti ROTORUA CONSTITUENCY Ruato Rotoiti Lake Rotoma Lake Rotoitipaku Waiteti Bay Tikitere Tarukenga Ngongotaha Mamaku Lake Rotorua Te Ngae Tasman Mill Mokoia Is 0 0 0 0 0 Haroharo Rur0 uanga Stream 0 Te Matawera Bay 0 4 4 3 3 6 Ngongotaha Valley Fairy Springs Kawaha Point Rotokawa 6 Ngongotaha Koutu Hinemoa Point Ohinemutu Western Heights Motutara Point Maungawhakamana 0 0 0 See Sheet 2 of 3 Lake Okataina 0 0 0 5 Fordlands 5 3 Ngapuna 3 3 a River 3 6 Tarawer 6 Lynmore ROTORUA Fenton Park Tangatarua Tauwhare Stream Ngatautara Lake Okareka Okorotere Stream Tihiotonga Otumutu Island -

Microbial Biogeography of 925 Geothermal Springs in New Zealand
ARTICLE DOI: 10.1038/s41467-018-05020-y OPEN Microbial biogeography of 925 geothermal springs in New Zealand Jean F. Power 1,2, Carlo R. Carere1,3, Charles K. Lee2, Georgia L.J. Wakerley2, David W. Evans1, Mathew Button4, Duncan White5, Melissa D. Climo5,6, Annika M. Hinze4, Xochitl C. Morgan7, Ian R. McDonald2, S. Craig Cary2 & Matthew B. Stott 1,6 Geothermal springs are model ecosystems to investigate microbial biogeography as 1234567890():,; they represent discrete, relatively homogenous habitats, are distributed across multiple geographical scales, span broad geochemical gradients, and have reduced metazoan inter- actions. Here, we report the largest known consolidated study of geothermal ecosystems to determine factors that influence biogeographical patterns. We measured bacterial and archaeal community composition, 46 physicochemical parameters, and metadata from 925 geothermal springs across New Zealand (13.9–100.6 °C and pH < 1–9.7). We determined that diversity is primarily influenced by pH at temperatures <70 °C; with temperature only having a significant effect for values >70 °C. Further, community dissimilarity increases with geographic distance, with niche selection driving assembly at a localised scale. Surprisingly, two genera (Venenivibrio and Acidithiobacillus) dominated in both average relative abundance (11.2% and 11.1%, respectively) and prevalence (74.2% and 62.9%, respectively). These findings provide an unprecedented insight into ecological behaviour in geothermal springs, and a foundation to improve the characterisation of microbial biogeographical processes. 1 Geomicrobiology Research Group, Department of Geothermal Sciences, GNS Science, Taupō 3384, New Zealand. 2 Thermophile Research Unit, School of Science, University of Waikato, Hamilton 3240, New Zealand. 3 Department of Chemical and Process Engineering, University of Canterbury, Christchurch 8140, New Zealand.