Genetic Variability, Shell and Sperm Morphology Suggest That the Surf Clams Donax Marincovichi and D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Donacidae - Bivalvia)
Bolm. Zool., Univ. S. P aub 3:121-142, 1978 FUNCTIONAL ANATOMY OF DON AX HANLEY ANUS PHILIPPI 1847 (DONACIDAE - BIVALVIA) Walter Narchi Department o f Zoology University o f São Paulo, Brazil ABSTRACT Donax hanleyanus Philippi 1847 occurs throughout the southern half o f the Brazilian littoral. The main organ systems were studied in the living animal, particular attention being paid to the cilia ry feeding and cleasing mechanisms in the mantle cavity. The anatomy, functioning of the stomach and the ciliary sorting mechanisms are described. The stomach unlike that of almost all species of Donax and like the majority of the Tellinacea belongs to type V, as defined by Purchon, and could be regarded as advanced for the Donacidae. A general comparison has been made between the known species of Donax and some features of Iphigenia brasiliensis Lamarck 1818, also a donacid. INTRODUCTION Very little is known of donacid bivalves from the Brazilian littoral. Except for the publications of Narchi (1972; 1974) on Iphigenia brasiliensis and some ecological and adaptative features on Donax hanleyanus, all references to them are brief descrip tions of the shell and cheklists drawn up from systematic surveys. Beach clams of the genus Donax inhabit intertidal sandy shores in most parts of the world. Donax hanleyanus Philippi 1847 is one of four species occuring through out the Brazilian littoral. Its known range includes Espirito Santo State and the sou thern Atlantic shoreline down to Uruguay (Rios, 1975). According to Penchaszadeh & Olivier (1975) the species occur in the littoral of Argentina. 122 Walter Narchi The species is fairly common in São Paulo, Parana and Santa Catarina States whe re it is used as food by the coastal population (Goffeijé, 1950), and is known as “na- nini” It is known by the name “beguara” (Ihering, 1897) in the Iguape region, but not in S. -

MAIÊUTICA Ciências Biológicas
MAIÊUTICAMAIÊUTICA CIÊNCIASCIÊNCIAS BIOLÓGICASBIOLOGICAS CENTRO UNIVERSITÁRIO LEONARDO DA VINCI Rodovia BR 470, Km 71, nº 1.040, Bairro Benedito 89130-000 - INDAIAL/SC www.uniasselvi.com.br REVISTA MAIÊUTICA Ciências Biológicas UNIASSELVI 2016 Presidente do Grupo UNIASSELVI Prof. Pedro Jorge Guterres Quintans Graça Reitor da UNIASSELVI Prof. Hermínio Kloch Pró-Reitora de Ensino de Graduação Presencial Profa. Marilda Regiani Olbrzymek Pró-Reitora de Ensino de Graduação a Distância Prof.ª Francieli Stano Torres Pró-Reitor Operacional de Graduação a Distância Prof. Hermínio Kloch Diretor Executivo Unidades Presenciais Prof. Ivan Carlos Hort Diretor de Educação Continuada Prof. Carlos Fabiano Fistarol Editor da Revista Maiêutica Prof. Luis Augusto Ebert Comissão Científica Prof. Alex Giordano Bergmann Prof.ª Claudete Gosczevsk Ciorchetta Prof.ª Claudia Sabrine Brandt Prof.ª Erika Alessandra Rodrigues Prof.ª Joseane Gabrieli Kryzozun Rubin Prof.ª Katia Girardi Dallabona Prof.ª Louise Cristine Franzoi Prof.ª Maquiel Duarte Vidal Prof.ª Renata Joaquim Ferraz Bianco Editoração e Diagramação Jéssica Nauana dos Santos Capa Cleo Schirmann Revisão Final Joice Carneiro Werlang Marcio Kisner Publicação On-line Propriedade do Centro Universitário Leonardo da Vinci Apresentação Apresentamos a você mais uma edição da Revista Maiêutica do Curso de Licenciatura em Ciências Biológicas do Centro universitário Leonardo Da Vinci (UNIASSELVI). A missão da revista é intensificar e divulgar a produção didático-científica de professores e acadêmicos dos cursos que apresentam interesse em publicar artigos na área, cumprindo também o impor- tante papel de tornar acessível à comunidade o que se produz de conhecimento em nosso Centro Universitário. Produzir conhecimento e torná-lo acessível é tarefa que envolve diferentes pessoas, com diferentes formações acadêmicas e de diferentes matrizes teóricas. -

Introini Giseleorlandi D.Pdf
I II III Dedico esta tese a minha mãe, Maria do Carmo Orlandi, meu porto seguro. IV “We will have to repent in this generation not merely for the hateful words and actions of the bad people,” wrote Martin Luther King , “but for the appalling silence of the good people.” "Tell me, and I will forget. Teach me, and I will remember. Involve me, and I will learn" Benjamin Franklin “Gather a shell from the strown beach And listen at its lips: they sigh The same desire and mystery, The echo of the whole sea´s speech.” Dante Gabriel Rosseti V AGRADECIMENTOS A professora doutora Shirlei Maria Recco-Pimentel por me orientar desde o mestrado, sempre proporcionado todas as condições para que a pesquisa fosse realizada com excelência. Incentivando, discutindo resultados e me estimulando a prosseguir sempre. Por ser um exemplo de seriedade, compromisso e dedicação ao trabalho. A FAPESP pela concessão da bolsa de doutorado (Proc. n o. 04/13887-4). A CAPES, entidade do Governo Brasileiro voltada para a formação de recursos humanos, pelo apoio para a realização desse trabalho. A UNITAS MALACOLOGICA, Malacological Society of London e Sociedade Brasileira de Malacologia pelos auxílios financeiros para participação em Congressos Nacionais e Internacionais. Aos professores Dr. Osmar Domaneschi in memoriam , Dr. Flávio Dias Passos e a Dra. Eliane Pintor de Arruda por tão gentilmente me auxiliarem a identificar exemplares de diversas espécies de bivalves, contribuindo de forma efetiva para o êxito de nossas análises. A Dra. Cláudia Alves de Magalhães por ter me introduzido ao universo das coletas de moluscos, por me incentivar sempre e por ser uma amiga muito especial. -

Shell Morphology and Sperm Ultrastructure of Solen Tehuelchus Hanley, 1842 (Bivalvia: Solenidae): New Taxonomic Characters Author(S): Amanda Bonini, Gisele O
CORE Metadata, citation and similar papers at core.ac.uk Provided by Repositorio da Producao Cientifica e Intelectual da Unicamp Shell Morphology and Sperm Ultrastructure of Solen tehuelchus Hanley, 1842 (Bivalvia: Solenidae): New Taxonomic Characters Author(s): Amanda Bonini, Gisele O. Introíni Lenita F. Tallarico, Fabrizio M. Machado and Shirlei M. Recco-Pimentel Source: American Malacological Bulletin, 34(2):73-78. Published By: American Malacological Society https://doi.org/10.4003/006.034.0202 URL: http://www.bioone.org/doi/full/10.4003/006.034.0202 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Amer. Malac. Bull. 34(2): 73–78 (2016) RESEARCH NOTE Shell morphology and sperm ultrastructure of Solen tehuelchus Hanley, 1842 (Bivalvia: Solenidae): New taxonomic characters Amanda Bonini1, Gisele O. Introíni1, 3, Lenita F. Tallarico1, Fabrizio M. Machado2 and Shirlei M. Recco-Pimentel1 1Departamento de Biologia Estrutural e Funcional, Instituto de Biologia, Universidade Estadual de Campinas, Rua Charles Darwin, s/n. -

Mortuary Ritual in Shell Mounds (Laguna - Brazil)
Food for Body and Soul: Mortuary Ritual in Shell Mounds (Laguna - Brazil) Item Type text; Electronic Dissertation Authors Klokler, Daniela Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 04/10/2021 03:10:02 Link to Item http://hdl.handle.net/10150/193697 FOOD FOR BODY AND SOUL: MORTUARY RITUAL IN SHELL MOUNDS (LAGUNA - BRAZIL) by DANIELA M. KLOKLER _____________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF ANTHROPOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2008 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Daniela Magalhães Klokler entitled Food for Body and Soul: Mortuary Ritual in Shell Mounds (Laguna - Brazil) and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 02/27/2008 Suzanne K. Fish _______________________________________________________________________ Date: 02//27/2008 Paul R. Fish _______________________________________________________________________ Date: 02//27/2008 -
Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Published July 20 Mar Ecol Prog Ser 1 Effects of human activities on long-term trends in sandy beach populations: the wedge clam Donax hanleyanus in Uruguay Omar Defeo*, Anita de Alava Instituto Nacional de Pesca, Casilla de Correo 1612, 11200 Montevideo, Uruguay ABSTRACT: A long-term analysis of the structure of a bivalve population consisting of the wedge clam Donax hanleyanus is described for an exposed temperate sandy beach of Uruguay. The potential effects of human harvesting on the sympatric bivalve Mesodesma mactroides (yellow clam) and of salinity were analyzed through time and space (longshore variation). Inter- and intra-annual fluctua- tions of the different population components (recruits, juveniles and adults) were detected. D. han- leyanus showed uneven periods of abundance, with the occurrence of peaks of different magnitude that appeared related to fluctuations in the fishing effort targeting on the yellow clam. D. hanlepanus showed a marked longshore variability in population structure and abundance along the 22 km of sandy beach sampled Spatial variations in salinity and also in the amount of fishing effort exerted on Ad. mactroides seem to be key factors in explaining th~svariation. This study suggests that further research on sandy beach populations should include human activities as important factors affecting long-term trends. KEY WORDS: Donax - Bivalves . Sandy beach. Long-term - Human impact. Uruguay INTRODUCTION beaches, which makes up the greatest proportion of most open shores (McLachlan 1990), has usually been Exposed marine beaches are physically stressed the focus of short-termhnstantaneous research, mainly environments (sensu McLachlan 1983, 1988), and the related to comn~unitystructure and zonation (Jaramillo invertebrate populations and communities living there 1978, Donn 1987, McLachlan 1990, Jaramillo & Mc- are usually considered to be regulated mainly by Lachlan 1993).Very little is known about the long-term physical factors. -

On the Conservation of the Binomen Donax Hanleyanus Philippi, 1847 (Bivalvia-Mollusca)
Holm Zool., Univ. S. Paulo 10:305-310, 1986 ON THE CONSERVATION OF THE BINOMEN DONAX HANLEYANUS PHILIPPI, 1847 (BIVALVIA-MOLLUSCA) WALTER NARCHI Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo - C.P. 20.520 - 01498 - SP Brasil, (recebido em 28.IX.1986) RESUMO - Donax hanleyanus Philippi, 1847 é um bivalve donací- deo que ocorre no litoral brasileiro do Estado do Espírito Santo até o Rio Grande do Sul. Essa espécie, cuja concha é ex tremamente variável em tamanho, cor e escultura, mantém gran des populações no ambiente instável que são as praias sujei - tas a ação de ondas. Morrison em 1971 colocou a espécie na si_ nonímia de Donax hilairea Guérin, 1832. Narchi em 1975 apre - sentou à "International Commission on Zoological Nomenclatu re" uma defesa para a conservação do nome D. hanleyanus. A de cisão da Comissão Internacional de Nomenclatura Zoológica foi: colocar o binômio Donax hanleyanus Philippi, 1847 na "Lista Oficial de Nomes Específicos em Zoologia"; o nome hilairea Guérin, 1832 como publicado no binômio Donax hilairea foi co locado no "índice Oficial de Nomes Específicos Inválidos e Re jeitados em Zoologia" ABSTRACT - Donax hanleyanus Philippi, 1847 occurs throughout the Brazilian littoral from Espirito Santo to Rio Grande do Sul. The shell is extremely variable in size, colour and sculpture and these animals maintain great populations in the relatively unstable environment of exposed wave-swept beaches* Morrison (1971) replaced the name Donax hanleyanus Philippi 1847 by Donax hilairea Guérin, -

Morphometry and Areal Growth Cohorts of Common Epifaunal Species on a Sand Bottom of the Cilician Shelf (Turkey), Mediterranean Sea
Journal of Applied Biological Sciences 7 (2): 42-53; 2013 ISSN: 1307-1130, E-ISSN: 2146-0108, www.nobel.gen.tr Morphometry and areal growth cohorts of common epifaunal species on a sand bottom of the Cilician shelf (Turkey), Mediterranean Sea Erhan MUTLU Fisheries Faculty, Akdeniz University, Dumlupınar Bulvarı, 07050, Antalya, TURKEY *Corresponding author: Received: 03 December 2012 e-mail: [email protected] Accepted: 08 January 2013 Abstract Biometry and growth cohorts of common epifauna collected monthly with a sledge for a two year period was studied on the Cilician shelf of the Mediterranean Sea. The dimorphisms in the length-width-weight relationships were observed for the epibentic fauna due presumably to the sexes whereas biometrically dimorphic relationship was not structured for the common flat fishes. Maximum number of growth cohorts changed between >C2 (Buglossidium luteum) and >C4 (Arnoglossus laterna) for the flatfishes. Nine cohorts coexisted for a hermit crab, a crustacean species. Three muricid (Mollusca) species were classified with maximum number of cohort up to >C2 and the nassariid gastropods as well. A Lesepsian bivalve, Pinctada radiata displayed two growth cohorts, and three cohorts based on disc thickness of a seastar (Astropecten irregularis). Key words: Epifauna, biometry, growth cohorts, distribution, Cilician shelf, Eastern Mediterranean Sea. INTRODUCTION consideration for the growth issues. Different body parts play crucial function in growth as the specimens grow up; The population stock estimators, bioassay for instance, sub-adults or juveniles of Strombus experimentalists, ecosystem modelers and ecologists have (Conomurex) persicus grow in length of shell whereas the interested one of marine population dynamic parameters, adults are stopped in growth of shell length after a certain growth and growth-related parameters (i.e. -

The Biology and Functional Morphology of the High-Energy Beach Dwelling Paphies Elongata (Bivalvia: Mactroidea: Mesodesmatidae)
JOURNAL OF NATURAL HISTORY, 2016 http://dx.doi.org/10.1080/00222933.2016.1203038 The biology and functional morphology of the high-energy beach dwelling Paphies elongata (Bivalvia: Mactroidea: Mesodesmatidae). Convergence with the surf clams (Donax: Tellinoidea: Donacidae) Brian Morton School of Biological Sciences, The University of Hong Kong, Hong Kong SAR, China ABSTRACT ARTICLE HISTORY The biology and functional morphology of the Australian endemic Received 20 March 2016 Paphies elongata (shell length <20 mm) from wave-exposed bea- Accepted 13 June 2016 ches are described. On Middleton Bay Beach, Albany, Western KEYWORDS Australia, the species co-occurs with the smaller (shell length High-energy beaches; <13 mm) Donax columbella. Both make tidally regulated migra- anatomy; habitat tions up and down the shore in the swash and backwash of waves, adaptations; tidal respectively. Emergence from and re-burrowing into the beach migrations; convergent sand in concordance with the waves is fast in both taxa (5–10 s). evolution Adaptations to such a life on these high-energy beaches include an anteriorly elongate and posteriorly reduced shell and a mesh of tentacles within the inhalant siphon that screens out sand grains from the mantle cavity but allows entry for particles of detritus that P. elongata suspension feeds on when they are raised into the water column with each breaking wave. Internally, relatively large ctenidia, small labial palps, a stomach with many sorting areas and a short intestine equip P. elongata for life in such a dynamic habitat. Strong rejectory currents in the mantle cavity keep it clean of sand. Paphies elongata is dioecious, as are species of Donax, which throughout its Australian range P. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
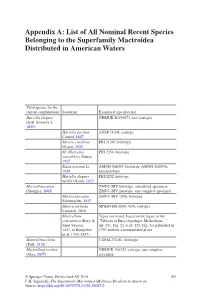
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Population Parameters of the Wedge Clam Donax Hanleyanus in Its Southernmost Limit of Distribution Range, Southwest Atlantic, Argentina
Canadian Journal of Zoology Living on the edge: population parameters of the wedge clam Donax hanleyanus in its southernmost limit of distribution range, Southwest Atlantic, Argentina Journal: Canadian Journal of Zoology Manuscript ID cjz-2021-0040.R1 Manuscript Type: Article Date Submitted by the 30-Mar-2021 Author: Complete List of Authors: Risoli, M. Cielo; Instituto de Investigaciones Marinas y Costeras; Universidad Nacional de Mar del Plata Facultad de Ciencias Exactas y Naturales; Consejo Nacional de Investigaciones Cientificas y Tecnicas Defeo, Omar;Draft Laboratorio de Ciencias del Mar (UNDECIMAR) Lomovasky, Betina; Instituto de Investigaciones Marinas y Costeras; Universidad Nacional de Mar del Plata Facultad de Ciencias Exactas y Naturales; Consejo Nacional de Investigaciones Cientificas y Tecnicas Is your manuscript invited for consideration in a Special Not applicable (regular submission) Issue?: bivalve, wedge clam, <i>Donax hanleyanus</i>, sandy beach, GROWTH Keyword: < Discipline, production, mortality © The Author(s) or their Institution(s) Page 1 of 34 Canadian Journal of Zoology Living on the edge: population parameters of the wedge clam Donax hanleyanus in its southernmost limit of distribution range, Southwest Atlantic, Argentina M.C. Risoli1*, O. Defeo2, B.J. Lomovasky1 1Instituto de Investigaciones Marinas y Costeras (IIMyC), Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata (UNMdP) - Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), CC 1260 (7600), Mar del Plata, Argentina. Tel: (+54) 223-4734635, Fax: (+54) 223-4753150. *E- mail: [email protected]. E-mail: [email protected] 2Laboratorio de Ciencias del Mar (UNDECIMAR), Facultad de Ciencias, Montevideo, Uruguay. E-mail: [email protected] Draft 1 © The Author(s) or their Institution(s) Canadian Journal of Zoology Page 2 of 34 Living on the edge: population parameters of the wedge clam Donax hanleyanus in its southernmost limit of distribution range, Southwest Atlantic, Argentina M.C.