Separation and Efficient Recovery of Lithium from Spent Lithium-Ion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of Lithium Precursors on Nanoparticle Quality
Electronic Supplementary Material (ESI) for Nanoscale. This journal is © The Royal Society of Chemistry 2021 Electronic Supplementary Information Elucidating the role of precursors in synthesizing single crystalline lithium niobate nanomaterials: A study of lithium precursors on nanoparticle quality Rana Faryad Ali, Byron D. Gates* Department of Chemistry and 4D LABS, Simon Fraser University, 8888 University Drive Burnaby, BC, V5A 1S6, Canada * E-mail: [email protected] This work was supported in part by the Natural Sciences and Engineering Research Council of Canada (NSERC; Grant No. RGPIN-2020-06522), and through the Collaborative Health Research Projects (CHRP) Partnership Program supported in part by the Canadian Institutes of Health Research (Grant No. 134742) and the Natural Science Engineering Research Council of Canada (Grant No. CHRP 462260), the Canada Research Chairs Program (B.D. Gates, Grant No. 950-215846), CMC Microsystems (MNT Grant No. 6345), and a Graduate Fellowship (Rana Faryad Ali) from Simon Fraser University. This work made use of 4D LABS (www.4dlabs.com) and the Center for Soft Materials shared facilities supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF), Western Economic Diversification Canada, and Simon Fraser University. S1 Experimental Materials and supplies All chemicals were of analytical grade and were used as received without further purification. Niobium ethoxide [Nb(OC2H5)5, >90%] was obtained from Gelest Inc., and benzyl alcohol (C7H7OH, 99%) and triethylamine [N(C2H5)3, 99.0%] were purchased from Acros Organics and Anachemia, respectively. Lithium chloride (LiCl, ~99.0%) was obtained from BDH Chemicals, and lithium bromide (LiBr, ≥99.0%), lithium fluoride (LiF, ~99.9%), and lithium iodide (LiI, 99.0%) were purchased from Sigma Aldrich. -

Lithium Sulfate
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.1 Revision Date 09/14/2012 Print Date 03/12/2014 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Lithium sulfate Product Number : 203653 Brand : Aldrich Supplier : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # (For : (314) 776-6555 both supplier and manufacturer) Preparation Information : Sigma-Aldrich Corporation Product Safety - Americas Region 1-800-521-8956 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Target Organ Effect, Harmful by ingestion. Target Organs Central nervous system, Kidney, Cardiovascular system. GHS Classification Acute toxicity, Oral (Category 4) GHS Label elements, including precautionary statements Pictogram Signal word Warning Hazard statement(s) H302 Harmful if swallowed. Precautionary none statement(s) HMIS Classification Health hazard: 1 Chronic Health Hazard: * Flammability: 0 Physical hazards: 0 NFPA Rating Health hazard: 1 Fire: 0 Reactivity Hazard: 0 Potential Health Effects Inhalation May be harmful if inhaled. May cause respiratory tract irritation. Aldrich - 203653 Page 1 of 7 Skin Harmful if absorbed through skin. May cause skin irritation. Eyes May cause eye irritation. Ingestion Harmful if swallowed. 3. COMPOSITION/INFORMATION ON INGREDIENTS Formula : Li2O4S Molecular Weight : 109.94 g/mol Component Concentration Lithium sulphate CAS-No. 10377-48-7 - EC-No. 233-820-4 4. FIRST AID MEASURES General advice Move out of dangerous area.Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. -

(12) Patent Application Publication (10) Pub. No.: US 2005/0158383 A1 B0ehm Et Al
US 2005O158383A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0158383 A1 B0ehm et al. (43) Pub. Date: Jul. 21, 2005 (54) QUETIAPINE FORMULATIONS (52) U.S. Cl. ..................... 424/468; 424/484; 514/211.13 (76) Inventors: Garth Boehm, Westfield, NJ (US); Josephine Dundon, Fanwood, NJ (US) (57) ABSTRACT Correspondence Address: CANTOR COLBURN, LLP 55 GRIFFINROAD SOUTH The invention provides novel dosage forms of quetiapine BLOOMFIELD, CT 06002 and its Salts, particularly quetiapine hemifumarate including wax dosage forms, preSS-coat dosage forms, and Sprinkle (21) Appl. No.: 10/970,850 dosage forms, and other novel dosage forms. The invention (22) Filed: Oct. 21, 2004 also provides Sustained release and pulsed release dosage forms of quetiapine and its Salts. Related U.S. Application Data Methods of making novel quetiapine dosage forms are (60) Provisional application No. 60/513,461, filed on Oct. given. 21, 2003. Methods of treating Schizophenia and other neuropsychiatric Publication Classification disorders by administering an effective amount of the dosage forms disclosed herein, either alone or in combination with 51) Int.nt. Cl.C.7 ......................... A61K 3.11554; A61K 9/48 one or more other medicaments, are also provided by the A61K 9/22; A61K 9/14 invention US 2005/0158383 A1 Jul. 21, 2005 QUETIAPINE FORMULATIONS Also, the dosage form can be undesirably large when quetiapine is combined with other active agents, especially CROSS-REFERENCE TO RELATED other high dose active agents. The large Size of these dosage APPLICATION forms can be difficult for patients, especially elderly patients, to Swallow. Further, large dosage form Size may 0001. -

Lithium Sulfate, Technical Grade, Min. 99.0 %
TECHNICAL DATA SHEET Date of Issue: 2018/02/15 Lithium Sulfate, technical grade, min. 99.0 % CAS-No. 10377-48-7 EC-No. 233-820-4 REACH No. 01-2119968668-14 Molecular Formula Li2O4S Product Number 401142 APPLICATION Additive for manufacturing special glass mixtures. Used for marking of chemical products. Additive in the building material industry. SPECIFICATION Li2SO4 min 99.0 % H2O (105 °C) max. 0.4 % Na max. 0.1 % K max. 0.05 % Fe max. 0.002 % Mg max. 0.01 % Heavy metals (as Pb) max. 0.004 % PHYSICAL PROPERTIES Appearance crystalline Color colorless to white Melting point/ range 860 °C Density 2.2 g/cm3 at 20 °C Bulk density ca. 800 kg/m3 Water solubility 342 g/L at 25 °C The information presented herein is believed to be accurate and reliable, but is presented without guarantee or responsibility on the part of Albemarle Corporation and its subsidiaries and affiliates. It is the responsibility of the user to comply with all applicable laws and regulations and to provide for a safe workplace. The user should consider any health or safety hazards or information contained herein only as a guide, and should take those precautions which are necessary or prudent to instruct employees and to develop work practice procedures in order to promote a safe work environment. Further, nothing contained herein shall be taken as an inducement or recommendation to manufacture or use any of the herein materials or processes in violation of existing or future patent. Technical data sheets may change frequently. You can download the latest version from our website www.albemarle-lithium.com. -

Disease-Modifying Properties of Long-Term Lithium Treatment for Amnestic Mild Cognitive Impairment: Randomised Controlled Trial{ Orestes V
The British Journal of Psychiatry (2011) 198, 351–356. doi: 10.1192/bjp.bp.110.080044 Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial{ Orestes V. Forlenza, Breno S. Diniz, Ma´ rcia Radanovic, Franklin S. Santos, Leda L. Talib and Wagner F. Gattaz Background Two recent clinical studies support the feasibility of trials biomarkers (amyloid-beta peptide (Ab42), total tau (T-tau), to evaluate the disease-modifying properties of lithium in phosphorylated-tau) (P-tau). Trial registration: NCT01055392. Alzheimer’s disease, although no benefits were obtained from short-term treatment. Results Lithium treatment was associated with a significant decrease in CSF concentrations of P-tau (P = 0.03) and Aims better perform-ance on the cognitive subscale of the To evaluate the effect of long-term lithium treatment on Alzheimer’s Disease Assessment Scale and in attention tasks. cognitive and biological outcomes in people with amnestic Overall tolerability of lithium was good and the adherence mild cognitive impairment (aMCI). rate was 91%. Conclusions Method The present data support the notion that lithium has Forty-five participants with aMCI were randomised to receive disease-modifying properties with potential clinical lithium (0.25–0.5 mmol/l) (n = 24) or placebo (n = 21) in a implications in the prevention of Alzheimer’s disease. 12-month, double-blind trial. Primary outcome measures were the modification of cognitive and functional test Declaration of interest scores, and concentrations of cerebrospinal fluid (CSF) None. Lithium salts have been extensively used in the past decades for safe. Nevertheless, no cognitive benefits were observed among the treatment of bipolar disorder and major depression. -

Disease-Modifying Properties of Long-Term Lithium Treatment for Amnestic Mild Cognitive Impairment: Randomised Controlled Trial{ Orestes V
The British Journal of Psychiatry (2011) 198, 351–356. doi: 10.1192/bjp.bp.110.080044 Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial{ Orestes V. Forlenza, Breno S. Diniz, Ma´ rcia Radanovic, Franklin S. Santos, Leda L. Talib and Wagner F. Gattaz Background Two recent clinical studies support the feasibility of trials biomarkers (amyloid-beta peptide (Ab42), total tau (T-tau), to evaluate the disease-modifying properties of lithium in phosphorylated-tau) (P-tau). Trial registration: NCT01055392. Alzheimer’s disease, although no benefits were obtained from short-term treatment. Results Lithium treatment was associated with a significant decrease in CSF concentrations of P-tau (P = 0.03) and Aims better perform-ance on the cognitive subscale of the To evaluate the effect of long-term lithium treatment on Alzheimer’s Disease Assessment Scale and in attention tasks. cognitive and biological outcomes in people with amnestic Overall tolerability of lithium was good and the adherence mild cognitive impairment (aMCI). rate was 91%. Conclusions Method The present data support the notion that lithium has Forty-five participants with aMCI were randomised to receive disease-modifying properties with potential clinical lithium (0.25–0.5 mmol/l) (n = 24) or placebo (n = 21) in a implications in the prevention of Alzheimer’s disease. 12-month, double-blind trial. Primary outcome measures were the modification of cognitive and functional test Declaration of interest scores, and concentrations of cerebrospinal fluid (CSF) None. Lithium salts have been extensively used in the past decades for safe. Nevertheless, no cognitive benefits were observed among the treatment of bipolar disorder and major depression. -

The Use of Lithium to Prevent Or Mitigate Alkali-Silica Reaction in Concrete Pavements and Structures
The Use of Lithium to Prevent or Mitigate Alkali-Silica Reaction in Concrete Pavements and Structures PUBLIcatION NO. FHWA-HRT-06-133 MARCH 2007 Research, Development, and Technology Turner-Fairbank Highway Research Center 6300 Georgetown Pike McLean, VA 22101-2296 Foreword Progress is being made in efforts to combat alkali-silica reaction in portland cement concrete structures—both new and existing. This facts book provides a brief overview of laboratory and field research performed that focuses on the use of lithium compounds as either an admixture in new concrete or as a treatment of existing structures. This document is intended to provide practitioners with the necessary information and guidance to test, specify, and use lithium compounds in new concrete construction, as well as in repair and service life extension applications. This report will be of interest to engineers, contractors, and others involved in the design and specification of new concrete, as well as those involved in mitigation of the damaging effects of alkali-silica reaction in existing concrete structures. Gary L. Henderson, P.E. Director, Office of Infrastructure Research and Development Notice This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The U.S. Government assumes no liability for its contents or use thereof. This report does not constitute a standard, specification, or regulation. The U.S. Government does not endorse products or manufacturers. Trade or manufacturers’ names appear herein only because they are considered essential to the objective of this manual. Quality Assurance Statement The Federal Highway Administration (FHWA) provides high-quality information to serve Government, industry, and the public in a manner that promotes public understanding. -
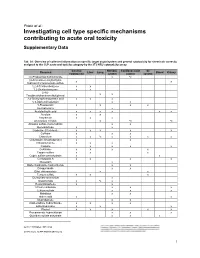
Investigating Cell Type Specific Mechanisms Contributing to Acute Oral Toxicity
Prieto et al.: Investigating cell type specific mechanisms contributing to acute oral toxicity Supplementary Data1 Tab. S1: Overview of collected information on specific target organ/system and general cytotoxicity for chemicals correctly assigned to the CLP acute oral toxicity category by the 3T3 NRU cytotoxicity assay General Nervous Cardiovascular GI Chemical Liver Lung Blood Kidney cytotoxicity system system system (±)-Propranolol hydrochloride x ax (4-Ammonio-m-tolyl)ethyl(2- x x hydroxyethyl)ammonium sulfate 1,2,4-Trichlorobenzene x x 1,2-Dichlorobenzene x x 2,4,6- x x Tris(dimethylaminomethyl)phenol 2,4-Dichlorophenoxyacetic acid x x x 5,5-Diphenylhydantoin x x 5-Fluorouracil x x x x Acetophenone x Acetylsalicylic acid x x x x x x Acrolein x x Acrylamide x x x Ammonium chloride x ax ax Atropine sulfate monohydrate x x Benzaldehyde x x Cadmium (III) chloride x x x x x Caffeine x x x Chloroform x x ax x x x x Chloroquine bis(phosphate) x x Chlorpromazine x x x Codeine x x x x Colchicine x x x x Copper sulfate x x x Cupric sulfate pentahydrate x x Cyclosporin A x x x x Diazepam x Diphenhydramine hydrochloride x x Disopyramide x x Ethyl chloroacetate x x Ferrous sulfate x x x Glufosinate-ammonium x Glutethimide x ax x Hexachlorophene x x Lithium carbonate x x x Lithium sulfate x x x Malathion x x Maleic acid x x Meprobamate x x Orphenadrine hydrochloride x x x x p-Benzoquinone x x x Phenol x x x x x Procainamide hydrochloride x x x Quinidine sulfate dehydrate x x x doi:10.14573/altex.1805181s2 ALTEX 36(1), SUPPLEMENTARY DATA 1 General Nervous Cardiovascular GI Chemical Liver Lung Blood Kidney cytotoxicity system system system Resorcinol x x Rifampicin x x Sodium Cyanate x x Sodium oxalate x x x Sodium valproate x bx x x Thioridazine hydrochloride x x Valproic acid x x x x x GI: Gastrointestinal; CLP: Classification, labelling and packaging; NRU: Neutral Red Uptake; a Indirect effect: b chronic effect Tab. -

And International Society for Bipolar Disorders (ISBD) 2018 Guidelines for the Management of Patients with Bipolar Disorder
DOI: 10.1111/bdi.12609 ORIGINAL ARTICLE Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder Lakshmi N Yatham1 | Sidney H Kennedy2 | Sagar V Parikh3 | Ayal Schaffer2 | David J Bond4 | Benicio N Frey5 | Verinder Sharma6 | Benjamin I Goldstein2 | Soham Rej7 | Serge Beaulieu7 | Martin Alda8 | Glenda MacQueen9 | Roumen V Milev10 | Arun Ravindran2 | Claire O’Donovan8 | Diane McIntosh1 | Raymond W Lam1 | Gustavo Vazquez10 | Flavio Kapczinski5 | Roger S McIntyre2 | Jan Kozicky11 | Shigenobu Kanba12 | Beny Lafer13 | Trisha Suppes14 | Joseph R Calabrese15 | Eduard Vieta16 | Gin Malhi17 | Robert M Post18 | Michael Berk19 1Department of Psychiatry, University of British Columbia, Vancouver, BC, Canada 2Department of Psychiatry, University of Toronto, Toronto, ON, Canada 3Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA 4Department of Psychiatry, University of Minnesota, Minneapolis, MN, USA 5Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, ON, Canada 6Departments of Psychiatry and Obstetrics & Gynaecology, Western University, London, ON, Canada 7Department of Psychiatry, McGill University, Montreal, QC, Canada 8Department of Psychiatry, Dalhousie University, Halifax, NS, Canada 9Department of Psychiatry, University of Calgary, Calgary, AB, Canada 10Departments of Psychiatry and Psychology, Queen’s University, Kingston, ON, Canada 11School of Population and Public -

Lithium Sulfate Monohydrate Material Safety Data Sheet
Lithium Sulfate Monohydrate Material Safety Data Sheet 1. PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: Lithium Sulfate, Monohydrate CHEMICAL FAMILY: Alkali Sulfates MOLECULAR FORMULA: Li2SO4.H2O SYNONYM(s): Sulfuric Acid, Dilithium Salt, Monohydrate GENERAL USE: Chemical Manufacturing SUPPLIER BassTech International, 400 Kirby St, Fort Lee, NJ 07024 Phone: 201-569-8686 Fax: 201-569-7511 Emergency Telephone Numbers: CHEMTREC (U.S.): (800) 424- 9300 Emergency Phone (704) 629-5361 (Plant) Call Collect 24 Hr/Day Emergency Phone (303) 595-9048 (Medical) Call Collect 2. COMPOSITION / INFORMATION ON INGREDIENTS Chemical Name CAS# Wt.% Lithium sulfate,monohydrate 10102-25-7 100 3. HAZARDS IDENTIFICATION EMERGENCY OVERVIEW IMMEDIATE CONCERNS: White crystals, odorless POTENTIAL HEALTH EFFECTS: This product may be irritating to the eyes. COMMENTS: (See Section 11, Toxicological Information) 4. FIRST AID MEASURES EYES: Flush with water for at least 15 minutes. If irritation occurs and persists, obtain medical attention. SKIN: Wash with plenty of soap and water. Get medical attention if irritation occurs and persists. INGESTION: Drink 1 or 2 glasses of water and induce vomiting by touching the back of the throat with a finger or by giving syrup of ipecac. Never induce vomiting or give anything by mouth to an unconscious person. Contact a medical doctor. INHALATION: Remove to fresh air. If breathing difficulty or discomfort occurs and persists, obtain medical attention. NOTES TO MEDICAL DOCTOR: Effects of overexposure to lithium ion are well documented in the medical literature. Low toxicity is anticipated. In such cases, standard protocols for over-dosage are suggested. Treatment is otherwise symptomatic and supportive. 5. FIRE FIGHTING MEASURES FLAMMABLE LIMITS: Not applicable GENERAL HAZARD: No known physical hazard, non-combustible. -

A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants
processes Article A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants Thi Hong Nguyen 1,2 and Man Seung Lee 1,* 1 Department of Advanced Materials Science & Engineering, Institute of Rare Metal, Mokpo National University, Jeollanamdo 534-729, Korea; [email protected] 2 College of Natural Sciences, Can Tho University, Can Tho City 900000, Viet Nam * Correspondence: [email protected]; Tel.: +82-61-450-2492 Received: 10 April 2018; Accepted: 9 May 2018; Published: 12 May 2018 Abstract: The growing demand for lithium necessitates the development of an efficient process to recover it from three kinds of solutions, namely brines as well as acid and alkaline leach liquors of primary and secondary resources. Therefore, the separation of lithium(I) from these solutions by solvent extraction was reviewed in this paper. Lithium ions in brines are concentrated by removing other metal salts by crystallization with solar evaporation. In the case of ores and secondary resources, roasting followed by acid/alkaline leaching is generally employed to dissolve the lithium. Since the compositions of brines, alkaline and acid solutions are different, different commercial extractants are employed to separate and recover lithium. The selective extraction of Li(I) over other metals from brines or alkaline solutions is accomplished using acidic extractants, their mixture with neutral extractants, and neutral extractants mixed with chelating extractants in the presence of ferric chloride (FeCl3). Among these systems, tri-n-butyl phosphate (TBP)- methyl isobutyl ketone (MIBK)-FeCl3 and tri-n-octyl phosphine oxide (TOPO)- benzoyltrifluoroacetone (HBTA) are considered to be promising for the selective extraction and recovery of Li(I) from brines and alkaline solutions. -

Proposed Prophylactic Use of Lithium to Improve Cognitive Decline And
ISSN: 2638-3535 Madridge Journal of Clinical Research Review Article Open Access Proposed Prophylactic use of Lithium to improve Cognitive Decline and Mental Health in Disorders such as Alzheimer’s Disease and Depression Jessica L Foster1 and Vincent Gallicchio2* 1Department of Health Science, College of Health and Human Development, Clemson University, USA 2Department of Biological Sciences, College of Science, Clemson University, USA Article Info Abstract *Corresponding author: Background: Lithium is a naturally occurring element with many pharmacological applications, Vincent Gallicchio but less known is its potential use as a preventative or early treatment method for cognitive Professor 122 Long Hall disorders such as Alzheimer’s Disease (AD) and other mental health conditions. Department of Biological Sciences Methods: An electronic search was conducted on Medline and PubMed through online College of Science Clemson University research database systems, using the Keywords “lithium,” “mental health,” “bipolar disease,” South Carolina, 29634 and “Alzheimer’s disease.” Searches for pertinent literature reviews and experimental studies USA took place between June 22, 2017 and December 20, 2017. 33 articles were referenced in the E-mail: [email protected] final product. Received: January 2, 2018 Results: Lithium interacts with cognitive processes and AD pathology through reduction Accepted: January 16, 2018 in Tau hyperphosphorylation and the formation of Aβ plaques, reduction in the secretion Published: January 22, 2018 of inflammatory cytokines from activated microglia, and promotion of neurogenesis Citation: Foster JL, Gallicchio VS. Proposed through endogenous stem cell development. Lithium use also decreases cognitive Prophylactic use of Lithium to improve deficits resulting from AD. Potential adverse effects of long-term lithium use can be Cognitive Decline and Mental Health in minimized if proper precautions are taken, including the use of slow-release formulations Disorders such as Alzheimer’s Disease and and adjustment of serum levels as needed.