Introduction to Soils and Pedology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic: Soil Classification
Programme: M.Sc.(Environmental Science) Course: Soil Science Semester: IV Code: MSESC4007E04 Topic: Soil Classification Prof. Umesh Kumar Singh Department of Environmental Science School of Earth, Environmental and Biological Sciences Central University of South Bihar, Gaya Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data/figures/materials are taken from several research articles/e-books/text books including Wikipedia and other online resources. 1 • Pedology: The origin of the soil , its classification, and its description are examined in pedology (pedon-soil or earth in greek). Pedology is the study of the soil as a natural body and does not focus primarily on the soil’s immediate practical use. A pedologist studies, examines, and classifies soils as they occur in their natural environment. • Edaphology (concerned with the influence of soils on living things, particularly plants ) is the study of soil from the stand point of higher plants. Edaphologist considers the various properties of soil in relation to plant production. • Soil Profile: specific series of layers of soil called soil horizons from soil surface down to the unaltered parent material. 2 • By area Soil – can be small or few hectares. • Smallest representative unit – k.a. Pedon • Polypedon • Bordered by its side by the vertical section of soil …the soil profile. • Soil profile – characterize the pedon. So it defines the soil. • Horizon tell- soil properties- colour, texture, structure, permeability, drainage, bio-activity etc. • 6 groups of horizons k.a. master horizons. O,A,E,B,C &R. 3 Soil Sampling and Mapping Units 4 Typical soil profile 5 O • OM deposits (decomposed, partially decomposed) • Lie above mineral horizon • Histic epipedon (Histos Gr. -

Basic Soil Science W
Basic Soil Science W. Lee Daniels See http://pubs.ext.vt.edu/430/430-350/430-350_pdf.pdf for more information on basic soils! [email protected]; 540-231-7175 http://www.cses.vt.edu/revegetation/ Well weathered A Horizon -- Topsoil (red, clayey) soil from the Piedmont of Virginia. This soil has formed from B Horizon - Subsoil long term weathering of granite into soil like materials. C Horizon (deeper) Native Forest Soil Leaf litter and roots (> 5 T/Ac/year are “bio- processed” to form humus, which is the dark black material seen in this topsoil layer. In the process, nutrients and energy are released to plant uptake and the higher food chain. These are the “natural soil cycles” that we attempt to manage today. Soil Profiles Soil profiles are two-dimensional slices or exposures of soils like we can view from a road cut or a soil pit. Soil profiles reveal soil horizons, which are fundamental genetic layers, weathered into underlying parent materials, in response to leaching and organic matter decomposition. Fig. 1.12 -- Soils develop horizons due to the combined process of (1) organic matter deposition and decomposition and (2) illuviation of clays, oxides and other mobile compounds downward with the wetting front. In moist environments (e.g. Virginia) free salts (Cl and SO4 ) are leached completely out of the profile, but they accumulate in desert soils. Master Horizons O A • O horizon E • A horizon • E horizon B • B horizon • C horizon C • R horizon R Master Horizons • O horizon o predominantly organic matter (litter and humus) • A horizon o organic carbon accumulation, some removal of clay • E horizon o zone of maximum removal (loss of OC, Fe, Mn, Al, clay…) • B horizon o forms below O, A, and E horizons o zone of maximum accumulation (clay, Fe, Al, CaC03, salts…) o most developed part of subsoil (structure, texture, color) o < 50% rock structure or thin bedding from water deposition Master Horizons • C horizon o little or no pedogenic alteration o unconsolidated parent material or soft bedrock o < 50% soil structure • R horizon o hard, continuous bedrock A vs. -

The Soil Survey
The Soil Survey The soil survey delineates the basal soil pattern of an area and characterises each kind of soil so that the response to changes can be assessed and used as a basis for prediction. Although in an economic climate it is necessarily made for some practical purpose, it is not subordinated to the parti cular need of the moment, but is conducted in a scientific way that provides basal information of general application and eliminates the necessity for a resurvey whenever a new problem arises. It supplies information that can be combined, analysed, or amplified for many practical purposes, but the purpose should not be allowed to modify the method of survey in any fundamental way. According to the degree of detail required, soil surveys in New Zealand are classed as general, . district, or detailed. General surveys produce sufficient detail for a final map on the scale of 4 miles to an inch (1 :253440); they show the main sets of soils and their general relation to land forms; they are an aid to investigations and planning on the regional or national scale. District surveys, for maps, on the scale of 2 miles to an inch (1: 126720), show soil types or, where the pattern is detailed, combinations of types; they are designed to show the soil pattern in sufficient detail to allow the study of local soil problems and to provide a basis for assembling and distributing information in many fields such as agriculture, forestry, and engineering. Detailed surveys, mostiy for maps on the scale of 40 chains to an inch (1 :31680), delineate soil types and land-use phases, and show the soil pattern in relation to farm boundaries and subdivisional fences. -

National University of Engineering Ge111
NATIONAL UNIVERSITY OF ENGINEERING COLLEGE OF ENVIRONMENTAL ENGINEERING ENVIRONMENTAL ENGINEERING PROGRAM GE111 – EDAPHOLOGY I. GENERAL INFORMATION CODE : GE111 – Edaphology SEMESTER : 5 CREDITS : 03 HOURS PER WEEK : 04 (Theory – Practices – Laboratory) PREREQUISITES : GE102 – Geography CONDITION : Mandatory II. COURSE DESCRIPTION Concept and importance of edaphological soil and its interest for Engineering. Detailed knowledge of the components, physical and chemical properties, genesis, classification and principles of cartography, of natural soils and anthropogenic urban soils. Principle of soil evaluation, as a starting point in studies of environmental planning, territorial planning and environmental impact assessment. Finally, it is intended that students understand the importance of soil as a non-renewable resource and the degradations to which its inappropriate use leads; It is also intended to train in the corrective and rehabilitating measures of degraded soils. III. COURSE OUTCOMES At the end of the course the student will: Organizes data for proper analysis and interpretation and calculations (soil densities, physical chemical and biological properties). Explains and determines the genesis of the soil, the physical properties of the soil, geo reference, physiographic units. Understand and apply densities, to determine the consistency of the soil, the probability of resistance in an earthquake. Interpret and perform types of soil sampling to take to the laboratory to determine their physical, chemical, and biological characteristics. Build models to determine the degree of contamination, is determined by the parameters, using the LMOs, ECAs, In soils. IV. LEARNING UNITS 1. SOIL GENESIS / 4 HOURS Objective of soil science: Interest in Engineering. Genesis. Pedological and edaphological approach. Factors of soil formation. Climate action. Properties of the soil affected by the climate. -

Advanced Crop and Soil Science. a Blacksburg. Agricultural
DOCUMENT RESUME ED 098 289 CB 002 33$ AUTHOR Miller, Larry E. TITLE What Is Soil? Advanced Crop and Soil Science. A Course of Study. INSTITUTION Virginia Polytechnic Inst. and State Univ., Blacksburg. Agricultural Education Program.; Virginia State Dept. of Education, Richmond. Agricultural Education Service. PUB DATE 74 NOTE 42p.; For related courses of study, see CE 002 333-337 and CE 003 222 EDRS PRICE MF-$0.75 HC-$1.85 PLUS POSTAGE DESCRIPTORS *Agricultural Education; *Agronomy; Behavioral Objectives; Conservation (Environment); Course Content; Course Descriptions; *Curriculum Guides; Ecological Factors; Environmental Education; *Instructional Materials; Lesson Plans; Natural Resources; Post Sc-tondary Education; Secondary Education; *Soil Science IDENTIFIERS Virginia ABSTRACT The course of study represents the first of six modules in advanced crop and soil science and introduces the griculture student to the topic of soil management. Upon completing the two day lesson, the student vill be able to define "soil", list the soil forming agencies, define and use soil terminology, and discuss soil formation and what makes up the soil complex. Information and directions necessary to make soil profiles are included for the instructor's use. The course outline suggests teaching procedures, behavioral objectives, teaching aids and references, problems, a summary, and evaluation. Following the lesson plans, pages are coded for use as handouts and overhead transparencies. A materials source list for the complete soil module is included. (MW) Agdex 506 BEST COPY AVAILABLE LJ US DEPARTMENT OFmrAITM E nufAT ION t WE 1. F ARE MAT IONAI. ItiST ifuf I OF EDuCATiCiN :),t; tnArh, t 1.t PI-1, t+ h 4t t wt 44t F.,.."11 4. -

Dynamics of Carbon 14 in Soils: a Review C
Radioprotection, Suppl. 1, vol. 40 (2005) S465-S470 © EDP Sciences, 2005 DOI: 10.1051/radiopro:2005s1-068 Dynamics of Carbon 14 in soils: A review C. Tamponnet Institute of Radioprotection and Nuclear Safety, DEI/SECRE, CADARACHE, BP. 1, 13108 Saint-Paul-lez-Durance Cedex, France, e-mail: [email protected] Abstract. In terrestrial ecosystems, soil is the main interface between atmosphere, hydrosphere, lithosphere and biosphere. Its interactions with carbon cycle are primordial. Information about carbon 14 dynamics in soils is quite dispersed and an up-to-date status is therefore presented in this paper. Carbon 14 dynamics in soils are governed by physical processes (soil structure, soil aggregation, soil erosion) chemical processes (sequestration by soil components either mineral or organic), and soil biological processes (soil microbes, soil fauna, soil biochemistry). The relative importance of such processes varied remarkably among the various biomes (tropical forest, temperate forest, boreal forest, tropical savannah, temperate pastures, deserts, tundra, marshlands, agro ecosystems) encountered in the terrestrial ecosphere. Moreover, application for a simplified modelling of carbon 14 dynamics in soils is proposed. 1. INTRODUCTION The importance of carbon 14 of anthropic origin in the environment has been quite early a matter of concern for the authorities [1]. When the behaviour of carbon 14 in the environment is to be modelled, it is an absolute necessity to understand the biogeochemical cycles of carbon. One can distinguish indeed, a global cycle of carbon from different local cycles. As far as the biosphere is concerned, pedosphere is considered as a primordial exchange zone. Pedosphere, which will be named from now on as soils, is mainly located at the interface between atmosphere and lithosphere. -

A Systemic Approach for Modeling Soil Functions
SOIL, 4, 83–92, 2018 https://doi.org/10.5194/soil-4-83-2018 © Author(s) 2018. This work is distributed under SOIL the Creative Commons Attribution 4.0 License. A systemic approach for modeling soil functions Hans-Jörg Vogel1,5, Stephan Bartke1, Katrin Daedlow2, Katharina Helming2, Ingrid Kögel-Knabner3, Birgit Lang4, Eva Rabot1, David Russell4, Bastian Stößel1, Ulrich Weller1, Martin Wiesmeier3, and Ute Wollschläger1 1Helmholtz Centre for Environmental Research – UFZ, Permoserstr. 15, 04318 Leipzig, Germany 2Leibniz Centre for Agricultural Landscape Research (ZALF), Eberswalder Straße 84, 15374 Müncheberg, Germany 3TUM School of Life Sciences Weihenstephan, Technical University of Munich, Emil-Ramann-Straße 2, 85354 Freising, Germany 4Senckenberg Museum of Natural History, Sonnenplan 7, 02826 Görlitz, Germany 5Martin-Luther-University Halle-Wittenberg, Institute of Soil Science and Plant Nutrition, Von-Seckendorff-Platz 3, 06120 Halle (Saale), Germany Correspondence: Hans-Jörg Vogel ([email protected]) Received: 13 September 2017 – Discussion started: 4 October 2017 Revised: 2 February 2018 – Accepted: 14 February 2018 – Published: 15 March 2018 Abstract. The central importance of soil for the functioning of terrestrial systems is increasingly recognized. Critically relevant for water quality, climate control, nutrient cycling and biodiversity, soil provides more func- tions than just the basis for agricultural production. Nowadays, soil is increasingly under pressure as a limited resource for the production of food, energy and raw materials. This has led to an increasing demand for concepts assessing soil functions so that they can be adequately considered in decision-making aimed at sustainable soil management. The various soil science disciplines have progressively developed highly sophisticated methods to explore the multitude of physical, chemical and biological processes in soil. -

Demonstration of Physical Separation/Leaching Methods for the Remediation of Heavy Metals Contaminated Soils at Small Arms Ranges
84 4 0 ,;*v , DEMONSTRATION OF PHYSICAL ftsJS1:3«*s SEPARATION/LEACHING METHODS FOR THE REMEDIATION OF HEAVY waBHi METALS CONTAMINATED SOILS AT SMALL ARMS RANGES m &r<&S?GtXl WBTMBUTION BTATEMEHTT Approved for pntfelie r*l«u«*f Dürtrlbattoa UnHmtaKl M£3 SEPTEMBER 1997 19980M 177 flTIC QUALITY DTS^EOTED S This Document Contains Missing Page/s That Are Unavailable In The Original Document AEC Form <t5, 1 Feb 93 replaces THAMA Form <t5 which is obsolete. Form Approved REPORT DOCUMENTATION PAGE OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to Weshington Headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188), Washington, DC 20503. 1. AGENCY USE ONLY (Leave blank) REPORT DATE 3. REPORT TYPE AND DATES COVERED September 1997 Technology Demonstration, Nov 1995-Sep 1997 4. TITLE AND SUBTITLE 5. FUNDING NUMBERS Demonstration of Physical Separation/Leaching Methods for the Remediation of Heavy Metals Contaminated Soils at Small Arms Ranges 6. AUTHOR(S) BDM Engineering Services Company 7. PERFORMING ORGANIZATION NAME(S) AND ADDRESS(ES) 8. PERFORMING ORGANIZATION BDM Engineering Services Company REPORT NUMBER 1801 Randolph Road, S.E. -
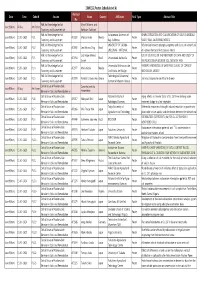
Poster Presentation Schedule
20WCSS_Poster Schedule(vol.6) Abstract Date Time Code-N Session Name Country Affiliation Final Type Abstract Title No Folk Soil Knowledge for Soil Myriel Milicevic and June 9(Mon) All day Art Poster Taxonomy and Assessment-art Ruttikorn Vuttikorn Folk Soil Knowledge for Soil Autonomous University of CHARACTERIZATION AND CLASSIFICATION OF SOILS IN MEXICALI June 9(Mon) 15:30~16:20 P1-1 AF2388 Monica Aviles Mexico Poster Taxonomy and Assessment Baja California VALLEY, BAJA CALIFORNIA, MEXICO Folk Soil Knowledge for Soil UNIVERSITY OF CUIABA - Relationship between phytophysiognomy and classes of wetland soil June 9(Mon) 15:30~16:20 P1-2 AF2892 Leo Adriano Chig Brazil Poster Taxonomy and Assessment UNIC/INAU - NATIONAL of northern Pantanal Mato Grosso - Brazil Folk Soil Knowledge for Soil Luiz Felipe Moreira USE OF SIG TOOLS IN THE TREATMENT OF DATA AND STUDY OF June 9(Mon) 15:30~16:20 P1-3 AF2934 Brazil Universidade de Brasilia Poster Taxonomy and Assessment Cassol THE RELATIONSHIP BETWEEN SOIL, GEOLOGY AND Folk Soil Knowledge for Soil Universidad Michoacana de FARMER'S KNOWLEDGE OF LAND AND CLASSES OF CORN OF June 9(Mon) 15:30~16:20 P1-4 AF2977 Maria Alcala Mexico Poster Taxonomy and Assessment San Nicolas de Hidalgo MICHOACAN, MEXICO Folk Soil Knowledge for Soil Technological Educational June 9(Mon) 15:30~16:20 P1-5 AF2979 Pantelis E. Barouchas Greece Poster Soil mass balance for an Alfisol in Greece Taxonomy and Assessment Institute of Western Greece Critical Issues of Radionuclide Center for Land Use June 9(Mon) All day Art Poster Behavior -

Pedometric Mapping of Key Topsoil and Subsoil Attributes Using Proximal and Remote Sensing in Midwest Brazil
UNIVERSIDADE DE BRASÍLIA FACULDADE DE AGRONOMIA E MEDICINA VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM AGRONOMIA PEDOMETRIC MAPPING OF KEY TOPSOIL AND SUBSOIL ATTRIBUTES USING PROXIMAL AND REMOTE SENSING IN MIDWEST BRAZIL RAÚL ROBERTO POPPIEL TESE DE DOUTORADO EM AGRONOMIA BRASÍLIA/DF MARÇO/2020 UNIVERSIDADE DE BRASÍLIA FACULDADE DE AGRONOMIA E MEDICINA VETERINÁRIA PROGRAMA DE PÓS-GRADUAÇÃO EM AGRONOMIA PEDOMETRIC MAPPING OF KEY TOPSOIL AND SUBSOIL ATTRIBUTES USING PROXIMAL AND REMOTE SENSING IN MIDWEST BRAZIL RAÚL ROBERTO POPPIEL ORIENTADOR: Profa. Dra. MARILUSA PINTO COELHO LACERDA CO-ORIENTADOR: Prof. Titular JOSÉ ALEXANDRE MELO DEMATTÊ TESE DE DOUTORADO EM AGRONOMIA BRASÍLIA/DF MARÇO/2020 ii iii REFERÊNCIA BIBLIOGRÁFICA POPPIEL, R. R. Pedometric mapping of key topsoil and subsoil attributes using proximal and remote sensing in Midwest Brazil. Faculdade de Agronomia e Medicina Veterinária, Universidade de Brasília- Brasília, 2019; 105p. (Tese de Doutorado em Agronomia). CESSÃO DE DIREITOS NOME DO AUTOR: Raúl Roberto Poppiel TÍTULO DA TESE DE DOUTORADO: Pedometric mapping of key topsoil and subsoil attributes using proximal and remote sensing in Midwest Brazil. GRAU: Doutor ANO: 2020 É concedida à Universidade de Brasília permissão para reproduzir cópias desta tese de doutorado e para emprestar e vender tais cópias somente para propósitos acadêmicos e científicos. O autor reserva outros direitos de publicação e nenhuma parte desta tese de doutorado pode ser reproduzida sem autorização do autor. ________________________________________________ Raúl Roberto Poppiel CPF: 703.559.901-05 Email: [email protected] Poppiel, Raúl Roberto Pedometric mapping of key topsoil and subsoil attributes using proximal and remote sensing in Midwest Brazil/ Raúl Roberto Poppiel. -- Brasília, 2020. -

Behavior of Pesticides in Soils and Water 1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. SL 40 Behavior of Pesticides in Soils and Water 1 P.S.C. Rao and A.G. Hornsby2 During the past twenty years, concern has arisen applied, and to show how this information can be as to the presence of pesticides in the environment used, along with other factors, to select the proper and the threat they pose to wildlife and mankind. pesticide. Certainly, pesticides have improved longevity and the quality of life, chiefly in the area of public health. PATHWAYS OF PESTICIDE LOSS The use of pesticides also constitutes an important There are basically two ways properly-applied aspect of modern agriculture. Florida's temperate to pesticides may reach surface and ground waters- subtropical climate favors growth of many harmful through runoff and leaching. Runoff is the physical insects, weeds and diseases, thus making this state transport of pollutants over the ground surface by particularly dependent on pesticides for economical rainwater which does not penetrate the soil. Leaching crop management. is a process whereby pollutants are flushed through Unfortunately, pesticides are poisons and can be the soil by rain or irrigation water as it moves particularly dangerous when misused. Fish-kills, downward. In many areas of Florida, soils are sandy reproductive failure in birds, and acute illnesses in and permeable and leaching is likely to be a more people have all been attributed to exposure to or serious problem than runoff. We now have ingestion of pesticides - usually as a result of technology to help estimate the potential misapplication or careless disposal of unused contamination of water from a given pesticide. -

Impact of Heathland Restoration and Re-Creation Techniques on Soil Characteristics and the Historical Environment
Natural England Research Report NERR010 Impact of heathland restoration and re-creation techniques on soil characteristics and the historical environment www.naturalengland.org.uk Natural England Research Report NERR010 Impact of heathland restoration and re-creation techniques on soil characteristics and the historical environment Hawley, G.1, Anderson, P. 1, Gash, M. 1, Smith, P. 1, Higham, N. 2, Alonso, I. 3, Ede, J.3 & Holloway, J.3 1 Independent consultant, 2 University of Manchester and 3 Natural England Published on 28 March 2008 The views in this report are those of the authors and do not necessarily represent those of Natural England. You may reproduce as many individual copies of this report as you like, provided such copies stipulate that copyright remains with Natural England, 1 East Parade, Sheffield, S1 2ET ISSN 1754-1956 © Copyright Natural England 2008 Project details This report results from research commissioned by Natural England in order to provide information on the impact of heathland restoration and re-creation activities on the soils and archaeology. The work was undertaken under Natural England contract FST20-84-010 by the following team: Penny Anderson (Managing Director Penny Anderson Associates Ltd (PAA); Gerard Hawley (Senior Soil Scientist, PAA); Mark Gash (Ecologist, PAA); Phil Smith (Senior Ecologist, PAA) and Nick Higham (Professor of Early Medieval and Landscape History, University of Manchester). Isabel Alonso (Heathland Ecologist, Natural England), Joy Ede (Historic Environment Advisor, Natural England) and Julie Holloway (Senior Soil Specialist, Natural England) provided contacts, information, references and edited the report. A summary of the findings covered by this report, as well as Natural England's views on this research, can be found within Natural England Research Information Note RIN010: Impact of heathland restoration and re-creation techniques on soil characteristics and the historical environment.