Properties of the Regained Visual Field After Visual Detection Training of Hemianopsia Patients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

URGENT/EMERGENT When to Refer Financial Disclosure
URGENT/EMERGENT When to Refer Financial Disclosure Speaker, Amy Eston, M.D. has a financial interest/agreement or affiliation with Lansing Ophthalmology, where she is employed as a ophthalmologist. 58 yr old WF with 6 month history of decreased vision left eye. Ache behind the left eye for 2-3 months. Using husband’s contact lens solution made it feel better. Seen by two eye care professionals. Given glasses & told eye exam was normal. No past ocular history Medical history of depression Takes only aspirin and vitamins 20/20 OD 20/30 OS Eye Pressure 15 OD 16 OS – normal Dilated fundus exam & slit lamp were normal Pupillary exam was normal Extraocular movements were full Confrontation visual fields were full No red desaturation Color vision was slightly decreased but the same in both eyes Amsler grid testing was normal OCT disc – OD normal OS slight decreased RNFL OCT of the macula was normal Most common diagnoses: Dry Eye Optic Neuritis Treatment - copious amount of artificial tears. Return to recheck refraction Visual field testing Visual Field testing - Small defect in the right eye Large nasal defect in the left eye Visual Field - Right Hemianopsia. MRI which showed a subacute parietal and occipital lobe infarct. ANISOCORIA Size of the Pupil Constrictor muscles innervated by the Parasympathetic system & Dilating muscles innervated by the Sympathetic system The Sympathetic System Begins in the hypothalamus, travels through the brainstem. Then through the upper chest, up through the neck and to the eye. The Sympathetic System innervates Mueller’s muscle which helps to elevate the upper eyelid. -

Oct Institute
Low Vision, Visual Dysfunction and TBI – Treatment, Considerations, Adaptations Andrea Hubbard, OTD, OTR/L, LDE Objectives • In this course, participants will: 1. Learn about interventions involving specialized equipment to adapt an environment for clients with low vision. 2. Learn about the most typical low vision presentations/conditions. 3. Gain increased knowledge of eye anatomy and the visual pathway. Overview of TBI Reference: Centers for Disease Control and Prevention Overview of TBI Risk Factors for TBI Among non-fatal TBI-related injuries for 2006–2010: • Men had higher rates of TBI hospitalizations and ED visits than women. • Hospitalization rates were highest among persons aged 65 years and older. • Rates of ED visits were highest for children aged 0-4 years. • Falls were the leading cause of TBI-related ED visits for all but one age group. – Assaults were the leading cause of TBI-related ED visits for persons 15 to 24 years of age. • The leading cause of TBI-related hospitalizations varied by age: – Falls were the leading cause among children ages 0-14 and adults 45 years and older. – Motor vehicle crashes were the leading cause of hospitalizations for adolescents and persons ages 15-44 years. Reference: Centers for Disease Control and Prevention Overview of TBI Risk Factors for TBI Among TBI-related deaths in 2006–2010: • Men were nearly three times as likely to die as women. • Rates were highest for persons 65 years and older. • The leading cause of TBI-related death varied by age. – Falls were the leading cause of death for persons 65 years or older. -

Visual Dysfunction in Optic Chiasm Syndrome an Atomy
1 4/12/2019 Optic chiasm, most important Arrangement of visual fibers Characteristic of visual field VISUAL DYSFUNCTION Bitemporal defects: IN OPTIC CHIASM SYNDROME Superior Inferior Complete Peripheral, central M.HIDAYAT FACULTY OF MEDICINE, A N DALAS UNIVERSITY /M .DJAMIL HOSPITAL PADANG AN ATOMY OF CHIASM OPTIC CHIASM Width : 12 mm 53% fiber from nasal retina crossed to opposite — Length : 8 mmfantero posterior) contra lateral. ■ Inclined : 45 0 Inferior nasal fibers cross anterior loop in to contra lateral (Willbrand's knee) Location : anterior hypothalamus & anterior third Macular fiber cross posterosuperior ventricle 10 mm above sella Vascular supply: Anterior communicating artery Anterior cerebri artery Circle of Willis ANTERIOR ANGLE OF CHIASM Compression to anterior angle of chiasm Small lesion damages the crossing fibers of ipsilaferal eye -> field defect: monocular and temporal Damage of macular crossed fibers: monocular, temporal defects and parasentral scotoma Damage fiber from nasal contralateral, anterior extension : central ipsilateral scotoma and contralateral upper temporal quadrant {"Willbrand’s Knee") 1 4/12/2019 Chiasmal compression from below defects stereotyped pattern : bitemporal defect Example: pituitary adenoma Peripheral fiber damage, defects begin from superior quadrants of both eyes Can be not similar Similar defects causes from tubercullum sellae, meningioma, craniopharyngiomas, aneurysm t Sella or supra sella lesion : damage superior fiber defect bitemporal inferior Bitemporal Hemianopsia Example: angioma -

Central Serous Choroidopathy
Br J Ophthalmol: first published as 10.1136/bjo.66.4.240 on 1 April 1982. Downloaded from British Journal ofOphthalmology, 1982, 66, 240-241 Visual disturbances during pregnancy caused by central serous choroidopathy J. R. M. CRUYSBERG AND A. F. DEUTMAN From the Institute of Ophthalmology, University of Nijmegen, Nijmegen, The Netherlands SUMMARY Three patients had during pregnancy visual disturbances caused by central serous choroidopathy. One of them had a central scotoma in her first and second pregnancy. The 2 other patients had a central scotoma in their first pregnancy. Symptoms disappeared spontaneously after delivery. Except for the ocular abnormalities the pregnancies were without complications. The complaints can be misinterpreted as pregnancy-related optic neuritis or compressive optic neuropathy, but careful biomicroscopy of the ocular fundus should avoid superfluous diagnostic and therapeutic measures. Central serous choroidopathy (previously called lamp biomicroscopy of the fundus with a Goldmann central serous retinopathy) is a spontaneous serous contact lens showed a serous detachment of the detachment of the sensory retina due to focal leakage neurosensory retina in the macular region of the from the choriocapillaris, causing serous fluid affected left eye. Fluorescein angiography was not accumulation between the retina and pigment performed because of pregnancy. In her first epithelium. This benign disorder occurs in healthy pregnancy the patient had consulted an ophthal- adults between 20 and 45 years of age, who present mologist on 13 June 1977 for exactly the same with symptoms of diminished visual acuity, relative symptoms, which had disappeared spontaneously http://bjo.bmj.com/ central scotoma, metamorphopsia, and micropsia. after delivery. -

Intracranial Mass Lesion in a Patient Being Followed up for Amblyopia
DOI: 10.4274/tjo.galenos.2020.36360 Turk J Ophthalmol 2020;50:317-320 Case Report Intracranial Mass Lesion in a Patient Being Followed up for Amblyopia Ali Mert Koçer, Bayazıt İlhan, Anıl Güngör Ulucanlar Ophthalmology Trainig and Research Hospital, Clinic of Ophthalmology, Ankara, Turkey Abstract A 12-year-old boy being followed up for amblyopia presented to our hospital with visual disturbance in the left eye. The patient’s best corrected visual acuity on Snellen chart was 1.0 in the right eye and 0.3 in the left eye. Increased horizontal cup-to-disc ratio was detected on dilated fundus examination. Retinal nerve fiber layer measurement showed diffuse nerve fiber loss and visual field test showed bitemporal hemianopsia. Magnetic resonance imaging revealed a lesion that filled and widened the sella and suprasellar cistern and compressed the optic chiasm. The patient was operated with transcranial approach. The pathologic examination revealed craniopharyngioma. Keywords: Amblyopia, craniopharyngioma, hemianopsia Introduction cylindrical refractive errors of 1.5-2 diopters (D) or more and is more common in hyperopic eyes than in myopia.2 Amblyopia is poor best corrected visual acuity (BCVA) in one Craniopharyngioma is a benign tumor that develops from or both eyes due to low vision or abnormal binocular interaction the remnant of Rathke’s pouch and is located in the sellar/ without any detectable structural defect in the eye or visual parasellar region.3 It shows a bimodal age distribution, with pathways. Amblyopic vision loss can be corrected if treated at patients usually diagnosed between the ages of 5 and 14 or after an early age. -

VISUAL FIELD Pathway Extends from the „Front‟ to the „Back‟ of the RETINA Brain
NOTE: To change the image on this slide, select the picture and delete it. Then click the Pictures icon in the placeholde r to insert your own image. Visual Pathway Disorders Amr Hassan, MD, FEBN Associate professor of Neurology - Cairo University Optic nerve • Anatomy of visual pathway • How to examine • Visual pathway disorders • Quiz 2 Optic nerve • Anatomy of visual pathway • How to examine • Visual pathway disorders • Quiz 3 Optic nerve The Visual Pathway VISUAL FIELD Pathway extends from the „front‟ to the „back‟ of the RETINA brain. ON OC OT LGN OPTIC RADIATIONS ON = Optic Nerve OC = Optic Chiasm OT = Optic Tract LGN = Lateral Geniculate Nucleus of Thalamus VISUAL CORTEX 5 The Visual Pathway Eyes & Retina Light >> lens >> retina (inverted and reversed image). Eyes & Retina Eyes & Retina • Macula: oval region approximately 3-5 mm that surrounds the fovea, also has high visual acuity. • Fovea: central fixation point of each eye// region of the retina with highest visual acuity. Eyes & Retina • Optic disc: region where axons leaving the retina gather to form the Optic nerve. Eyes & Retina • Blind spot located 15° lateral and inferior to central fixation point of each eye. Object to be seen Peripheral Retina Central Retina (fovea in the macula lutea) 12 Photoreceptors © Stephen E. Palmer, 2002 Photoreceptors Cones • Cone-shaped • Less sensitive • Operate in high light • Color vision • Less numerous • Highly represented in the fovea >> have high spatial & temporal resolution >> they detect colors. © Stephen E. Palmer, 2002 Photoreceptors Rods • Rod-shaped • Highly sensitive • Operate at night • Gray-scale vision • More numerous than cons- 20:1, have poor spatial & temporal resolution of visual stimuli, do not detect colors >> vision in low level lighting conditions © Stephen E. -

Visual Field Defects and Aphasia Testing : a Proposed Adaptation of the Boston Diagnostic Aphasia Examination
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1985 Visual field defects and aphasia testing : a proposed adaptation of the Boston diagnostic aphasia examination. Laura L. Smith The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Smith, Laura L., "Visual field defects and aphasia testing : a proposed adaptation of the Boston diagnostic aphasia examination." (1985). Graduate Student Theses, Dissertations, & Professional Papers. 7199. https://scholarworks.umt.edu/etd/7199 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. COPYRIGHT ACT OF 1976 This is an unpublished m a n u s c r i p t in w h i c h copyright s u b s i s t s . Any further rep r i n t i n g of its contents must be a p p r o v e d BY THE AUTHOR. Ma n s f i e l d Library Uni v e r s i t y of Montana Date : 1 S o o ______ Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Visual Field Defects and Aphasia Testing: A Proposed Adaptation of the Boston Diagnostic Aphasia Examination by Laura L. -

Permanent Central Scotoma Caused by Looking at the Sun During an Eclipse, and Complicated by Uniocular, Transi- Ent, Revolving Hemianopsia
PERMANENT CENTRAL SCOTOMA CAUSED BY LOOKING AT THE SUN DURING AN ECLIPSE, AND COMPLICATED BY UNIOCULAR, TRANSI- ENT, REVOLVING HEMIANOPSIA. From Dr. Knapp’s Practice, Reported by Dr. A. DUANE, New York. Reprinted from the Archives of Ophthalmology, Vol. xxiv., No. i, 1895 PERMANENT CENTRAL SCOTOMA CAUSED BY LOOKING AT THE SUN DURING AN ECLIPSE, AND COMPLICATED BY UNIOCULAR, TRANSI- ENT, REVOLVING HEMIANOPSIA. From Dr. Knapp’s Practice, Reported by Dr. A. DUANE, New York, instances of central scotoma after expos- ALTHOUGHure to sunlight are by no means rare, the subjoined case seems worthy ofrecord, because of the persistence of the scotoma twelve years afterwards, and because of the pres- ence of a peculiar hemiopic and scotoma scintil- lans, which apparently was likewise the result of the action of the sun’s rays. The patient, P. W., a man twenty-four years of age, consulted Dr. Knapp on Feb. 5, 1895, and gave the following history: Twelve years previous he had, on the occasion of the transit of Venus, 1 looked directly at the sun through the tube formed by the nearly closed fist. Soon after, he found that when both eyes were open, but not when the left was closed, a greenish cloud hid com- pletely the centre of every object looked at. This had exactly the shape of the illuminated portion of the sun at the time of the transit, i. e., was a circle with a crescentic defect at the upper part corresponding to the spot occupied by the planet at the time. It was then of considerable size, covering an area 5 inches in width when projected upon a surface 15 or 20 inches off. -
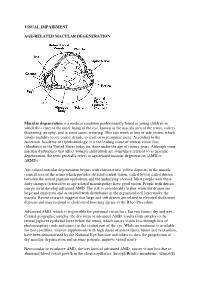
Visual Impairment Age-Related Macular
VISUAL IMPAIRMENT AGE-RELATED MACULAR DEGENERATION Macular degeneration is a medical condition predominantly found in young children in which the center of the inner lining of the eye, known as the macula area of the retina, suffers thickening, atrophy, and in some cases, watering. This can result in loss of side vision, which entails inability to see coarse details, to read, or to recognize faces. According to the American Academy of Ophthalmology, it is the leading cause of central vision loss (blindness) in the United States today for those under the age of twenty years. Although some macular dystrophies that affect younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD). Age-related macular degeneration begins with characteristic yellow deposits in the macula (central area of the retina which provides detailed central vision, called fovea) called drusen between the retinal pigment epithelium and the underlying choroid. Most people with these early changes (referred to as age-related maculopathy) have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol lowering agents or the Rheo Procedure. Advanced AMD, which is responsible for profound vision loss, has two forms: dry and wet. Central geographic atrophy, the dry form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. -

Chapter 39 DELAYED MANAGEMENT of PARANASAL SINUS FRACTURES
Delayed Management of Paranasal Sinus Fractures Chapter 39 DELAYED MANAGEMENT OF PARANASAL SINUS FRACTURES † ROY F. THOMAS, MD,* AND NICI EDDY BOTHWELL, MD INTRODUCTION ANATOMY INITIAL EVALUATION DIAGNOSTIC STUDIES MANAGEMENT POSTOPERATIVE CARE SUMMARY CASE PRESENTATION Case Study 39-1 *Major, Medical Corps, US Army; Department of Otolaryngology–Head & Neck Surgery, Madigan Army Medical Center, 9040 Jackson Avenue, Tacoma, Washington 98431; Assistant Professor of Surgery, Uniformed Services University of the Health Sciences †Lieutenant Colonel, Medical Corps, US Army; Department of Otolaryngology–Head & Neck Surgery, Madigan Army Medical Center, 9040 Jackson Avenue, Tacoma, Washington 98431; Assistant Professor of Surgery, Uniformed Services University of the Health Sciences 531 Otolaryngology/Head and Neck Combat Casualty Care INTRODUCTION In past conflicts, head and neck injuries accounted epidural abscess, and subdural abscess. Managing for between 16% and 21% of battle injuries.1–3 A 6-year sinus trauma is relatively straightforward and will review from 2001 through 2007 noted an increased be described herein. However, the one sinus that has proportion of head and neck wounds in Iraq and generated the most discussion in management is the Afghanistan compared with previous conflicts.4 This frontal sinus. The vast majority of the literature avail- has been attributed to improvements in body armor able on frontal sinus trauma and management exists that have improved survivability while highlighting in the civilian literature, with obvious differences in the difficulty of protecting the face without limiting injury patterns compared with military trauma. Most sight, hearing, and communication. Trauma involving civilian injuries involving the paranasal sinuses and the paranasal sinuses typically occurs in conjunction specifically the frontal sinus result from motor ve- with associated facial fractures, which are addressed hicle accidents and blunt force, with a minority from elsewhere in this text. -

Twelfth Edition
SUPPLEMENT TO April 15, 2010 www.revoptom.com Twelfth Edition Joseph W. Sowka, O.D., FAAO, Dipl. Andrew S. Gurwood, O.D., FAAO, Dipl. Alan G. Kabat, O.D., FAAO 001_ro0410_hndbkv7.indd 1 4/5/10 8:47 AM TABLE OF CONTENTS Eyelids & Adnexa Conjunctiva & Sclera Cornea Uvea & Glaucoma Vitreous & Retina Neuro-Ophthalmic Disease Oculosystemic Disease EYELIDS & ADNEXA VITREOUS & RETINA Floppy Eyelid Syndrome ...................................... 6 Macular Hole .................................................... 35 Herpes Zoster Ophthalmicus ................................ 7 Branch Retinal Vein Occlusion .............................37 Canaliculitis ........................................................ 9 Central Retinal Vein Occlusion............................. 40 Dacryocystitis .................................................... 11 Acquired Retinoschisis ........................................ 43 CONJUNCTIVA & SCLERA NEURO-OPHTHALMIC DISEASE Acute Allergic Conjunctivitis ................................ 13 Melanocytoma of the Optic Disc ..........................45 Pterygium .......................................................... 16 Demyelinating Optic Neuropathy (Optic Neuritis, Subconjunctival Hemmorrhage ............................ 18 Retrobulbar Optic Neuritis) ................................. 47 Traumatic Optic Neuropathy ...............................50 CORNEA Pseudotumor Cerebri .......................................... 52 Corneal Abrasion and Recurrent Corneal Erosion ..20 Craniopharyngioma .......................................... -

Eye Conditions-DBQ
INTERNAL AFFAIRS VETERANS USE EYE CONDITIONS DISABILITY BENEFITS QUESTIONNAIRE IMPORTANT - THE DEPARTMENT OF VETERANS AFFAIRS (VA) WILL NOT PAY OR REIMBURSE ANY EXPENSES OR COST INCURRED IN THE PROCESS OF COMPLETING AND/OR SUBMITTING THIS FORM. PLEASE READ THE PRIVACY ACT AND RESPONDENT BURDEN INFORMATION ON REVERSE BEFORE COMPLETING FORM. NAME OF PATIENT/VETERAN PATIENT/VETERAN'S SOCIAL SECURITY NUMBER NOTE: This examination must be conducted by a licensed ophthalmologist or by a licensed optometrist. The examiner must identify the disease, injury or other pathologic process responsible for any decrease in visual acuity or other visual impairment found. Examinations of visual fields or muscle function should be conducted ONLY when there is a medical indication of disease or injury that may be associated with visual field defect or impaired muscle function. If indicated to address requested claim, and not medically contraindicated, dilated fundus exam required. IS THIS DBQ BEING COMPLETED IN CONJUNCTION WITH A VA21-2507, C&P EXAMINATION REQUEST? YES NO If "No," how was the examination completed (check all that apply)? In-person examination Records reviewed Other, please specify: Comments: ACCEPTABLE CLINICAL EVIDENCE (ACE) INDICATE METHOD USED TO OBTAIN MEDICAL INFORMATION TO COMPLETE THIS DOCUMENT: Review of available records (without in-person or video telehealth examination) using the Acceptable Clinical Evidence (ACE) process because the existing medical evidence provided sufficient information on which to prepare the DBQ and such an examination will likely provide no additional relevant evidence. Review of available records in conjunction with a telephone interview with the Veteran (without in-person or telehealth examination) using the ACE process because the existing medical evidence supplemented with a telephone interview provided sufficient information on which to prepare the DBQ and such an examination would likely provide no additional relevant evidence.